UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 10-K
(Mark One)
|
☒ |
ANNUAL REPORT UNDER SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
For the fiscal year ended: May 31, 2019
|
☐ |
TRANSITION REPORT UNDER SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
For the transition period from ________ to ________
Commission File No.: 000-55546
CLS HOLDINGS USA, INC.
(Exact name of registrant as specified in its charter)
|
Nevada |
45-1352286 |
|
(State or other jurisdiction of incorporation or organization) |
(I.R.S. Employer Identification No.) |
11767 South Dixie Highway, Suite 115, Miami, Florida 33156
(Address of principal executive offices)
(888) 438-9132
(Registrant’s telephone number)
Securities registered under Section 12(b) of the Exchange Act:
|
Title of each class |
|
Trading Symbol(s) |
|
Name of each exchange on which registered |
|
N/A |
|
N/A |
|
N/A |
Securities registered under Section 12(g) of the Exchange Act:
Common Stock, par value $.0001
Indicate by check mark if the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act. ☐Yes ☒ No
Indicate by check mark if the registrant is not required to file reports pursuant to Section 13 or Section 15(d) of the Exchange Act. ☐ Yes ☒ No
Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. ☒ Yes ☐ No
Indicate by check mark whether the registrant has submitted electronically every Interactive Data File required to be submitted pursuant to Rule 405 of Regulation S-T (§ 232.405 of this chapter) during the preceding 12 months (or for such shorter period that the registrant was required to submit such files). ☒ Yes ☐ No
Indicate by check mark if disclosure of delinquent filers pursuant to Item 405 of Regulation S-K (§ 229.405 of this chapter) is not contained herein, and will not be contained, to the best of registrant’s knowledge, in definitive proxy or information statements incorporated by reference in Part III of this Form 10-K or any amendment to this Form 10-K. ☐
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, a smaller reporting company, or an emerging growth company. See the definitions of “large accelerated filer,” “accelerated filer,” “smaller reporting company,” and “emerging growth company” in Rule 12b-2 of the Exchange Act.
|
Large accelerated filer ☐ |
Accelerated filer ☐ |
|
Non-accelerated filer ☐ (Do not check if smaller reporting company) |
Smaller reporting company ☒ |
|
|
Emerging Growth Company ☐ |
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. ☐
Indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Exchange Act). ☐ Yes ☒ No
State the aggregate market value of the voting and non-voting common equity held by non-affiliates computed by reference to the price at which the common equity was last sold, or the average bid and asked price of such common equity, as of the last business day of the registrant’s most recently completed second fiscal quarter: $5,323,994.
Indicate the number of shares outstanding of each of the registrant’s classes of common stock, as of the latest practicable date: 126,420,345 shares of common stock, par value $0.0001, as of August 26, 2019.
DOCUMENTS INCORPORATED BY REFERENCE
None.
|
|
|
Page |
|
|
|
|
|
PART I |
|
|
|
|
|
|
|
Item 1. |
7 |
|
|
Item 1A. |
32 |
|
|
Item 2. |
54 |
|
|
Item 3. |
54 |
|
|
Item 4. |
54 |
|
|
|
|
|
|
PART II |
|
|
|
|
|
|
|
Item 5. |
55 |
|
|
Item 6. |
56 |
|
|
Item 7. |
Management’s Discussion and Analysis of Financial Condition and Results of Operations |
57 |
|
Item 7A. |
74 |
|
|
Item 8. |
75 |
|
|
Item 9. |
Changes in and Disagreements with Accountants on Accounting and Financial Disclosure |
76 |
|
Item 9A. |
76 |
|
|
Item 9B. |
76 |
|
|
|
|
|
|
PART III |
|
|
|
|
|
|
|
Item 10. |
77 |
|
|
Item 11. |
80 |
|
|
Item 12. |
Security Ownership of Certain Beneficial Owners and Management and Related Stockholder Matters |
83 |
|
Item 13. |
Certain Relationships and Related Transactions and Director Independence |
85 |
|
Item 14. |
87 |
|
|
|
|
|
|
PART IV |
|
|
|
|
|
|
|
Item 15. |
88 |
|
|
|
|
|
|
94 |
||
|
|
|
|
Cautionary Note Regarding Forward-Looking Statements
This annual report contains “forward-looking statements” within the meaning of within the meaning of Section 27A of the Securities Act of 1933, as amended, and Section 21E of the Securities Exchange Act of 1934, as amended, which is also referred to as “forward-looking information” that relate to the Company’s current expectations and views of future events. The forward-looking information is contained principally in the sections entitled “Our Business,” “Management’s Discussion and Analysis” and “Risk Factors”.
We have based these forward-looking statements largely on our current expectations and projections about future events and trends that we believe may affect our financial condition, results of operations, strategy, short-term and long-term business operations and objectives and financial needs.
In some cases, the forward-looking information can be identified by words or phrases such as “may”, “might”, “will”, “expect”, “anticipate”, “estimate”, “intend”, “plan”, “indicate”, “seek”, “believe”, “predict” or “likely”, or the negative of these terms, or other similar expressions intended to identify forward-looking information. The Company has based this forward-looking information on its current expectations and projections about future events and financial trends that it believes might affect its financial condition, results of operations, business strategy and financial needs. This forward-looking information includes, among other things, information and statements relating to:
|
|
● |
our expectations regarding our revenue, expenses and operations |
|
|
● |
our anticipated cash needs, our needs for additional financing, changes to our dividend policies |
|
|
● |
our intention to grow our business and our operations, including the addition of retail stores, grow operation expansion and the expansion of our production operation |
|
|
● |
our anticipated phases and timing of the expansion at the Warehouse Facility and the production capacity thereof |
|
|
● |
the expected growth in the number of consumers using our products |
|
|
● |
the expected growth of the cannabis industry in Nevada, Massachusetts and in the U.S. |
|
|
● |
our ability to finance our planned operations and proposed acquisitions, including the transactions contemplated by the IGH Option Agreement |
|
|
● |
medical benefits, viability, safety, efficacy and dosing of cannabis |
|
|
● |
expectations with respect to future production costs and capacity |
|
|
● |
expectations with respect to the renewal and/or extension of our licenses |
|
|
● |
expectations with respect to our plan to apply for additional retail store licenses |
|
|
● |
expectations with respect to the effects our patent will have on costs and revenues |
|
|
● |
market reception of our current product offerings and other new delivery mechanisms produced by us for use by consumers |
|
|
● |
our competitive position and the regulatory environment in which we operate |
|
|
● |
any commentary or legislative changes related to the legalization of medical or recreational cannabis and the timing related to such commentary or legalization |
|
|
● |
any changes to U.S. federal policies regarding the enforcement of the Controlled Substances Act |
Forward-looking information is based on certain assumptions and analyses made by the Company in light of the experience and perception of historical trends, current conditions and expected future developments and other factors it believes are appropriate, and is subject to risks and uncertainties. The material factors and assumptions used to make the forward-looking information include, among other things:
|
|
● |
our revenue, expenses and operations not being subject to a material adverse effect |
|
|
● |
our anticipated cash needs, our needs for additional financing, and changes to our dividend policies not being subject to a material adverse effect |
|
|
● |
our ability to grow the business and our operations, including the addition of retail stores and grow operation expansion, and the expansion of production and manufacturing at the Warehouse Facility occurring as currently planned |
|
|
● |
the price of cannabis and cannabis products not being materially adversely affected |
|
|
● |
there being no material adverse effect on our ability or capacity to produce cannabis or cannabis products |
|
|
● |
there being no material adverse effect in the anticipated phases and timing of the expansion at the Warehouse Facility and the production capacity thereof |
|
|
● |
the continued growth in the number of consumers using our products being as currently projected |
|
|
● |
the continued growth of the cannabis industry in Nevada, Massachusetts and the U.S. and there being no material adverse effect in the market for cannabis or the regulatory environment in Nevada, Massachusetts or at the federal level in the U.S. |
|
|
● |
there being no material adverse effect with respect to the medical benefits, viability, safety, efficacy and dosing of cannabis, including there being no loss of public trust in the medical benefits, viability, safety, efficacy and dosing of cannabis |
|
|
● |
there being no material adverse effect with respect to our expectations for future production costs and capacity |
|
|
● |
the Company being able to renew and/or extend its licenses |
|
|
● |
the Company being able to apply for additional retail store licenses |
|
|
● |
there being no material adverse effect with respect to our expectations on the effects our patent will have on costs and revenues |
|
|
● |
there remaining a positive market reception of our current product offerings and other new delivery mechanisms produced by the Company for use by consumers |
|
|
● |
the Company maintaining its competitive position and there being no material adverse effect in the regulatory environment in which the Company operates |
|
|
● |
there being no material adverse effect related to the legalization of medical or recreational cannabis and the timing related to such legalization |
Although we believe that the assumptions underlying this information is reasonable, they may prove to be incorrect, and we cannot assure that actual results will be consistent with this forward-looking information. Given these risks, uncertainties and assumptions, prospective investors should not place undue reliance on this forward-looking information. Whether actual results, performance or achievements will conform to our expectations and predictions is subject to a number of known and unknown risks, uncertainties, assumptions and other factors, including those listed under “Risk Factors”, which include:
|
|
● |
ongoing compliance with regulatory requirements relating to our business |
||
|
|
● |
changes in laws, regulations and guidelines relating to our business |
||
|
|
● |
difficulties in obtaining bank accounts and transferring money |
||
|
|
● |
risk of prosecution of the cannabis business at the federal level in the U.S. due to the ambiguity of laws in relation to medical cannabis and the cannabis business |
||
|
|
● |
accuracy of current research regarding the medical benefits, viability, safety, efficacy and dosing of cannabis |
||
|
|
● |
our history of losses |
||
|
|
● |
failure or delay in the growth of the business and our operations, including the addition of retail stores, grow operation expansion, and the Warehouse Facility Expansion |
||
|
|
● |
failure or delay in the anticipated phases and timing of the expansion at the Warehouse Facility and a consequently reduced production capacity |
||
|
|
● |
reliance on management and loss of members of management or other key personnel or an inability to attract new management team members |
||
|
|
● |
inability to raise financing to fund on-going operations, capital expenditures or acquisitions |
||
|
|
● |
inability to realize growth targets |
||
|
|
● |
requirements of additional financing |
||
|
|
● |
competition in our industry |
||
|
|
● |
inability to acquire and retain new clients |
||
|
|
● |
inability to develop new technologies and products and the obsolescence of existing technologies and products |
||
|
|
● |
vulnerability to rising energy costs |
||
|
|
● |
vulnerability to increasing costs and obligations related to investment in infrastructure, growth and regulatory compliance |
||
|
|
● |
dependence on third party transportation services to deliver our products |
||
|
|
● |
unfavorable publicity or consumer perception |
||
|
|
● |
product liability claims and product recalls |
||
|
|
● |
reliance on key inputs and their related costs |
||
|
|
● |
dependence on suppliers and skilled labor |
||
|
|
● |
difficulty associated with forecasting demand for products |
||
|
|
● |
operating risk and insurance coverage |
||
|
|
● |
inability to manage growth |
||
|
|
● |
conflicts of interest among our officers and directors |
||
|
|
● |
environmental regulations and risks |
||
|
|
● |
managing damage to our reputation and third party reputational risks |
||
|
|
● |
inability to adequately protect our intellectual property due to cannabis being illegal under U.S. federal law |
||
|
|
● |
potential reclassification/re-categorization of cannabis as a controlled substance in the U.S. |
||
|
|
● |
changes to safety, health and environmental regulations |
||
|
|
● |
exposure to information systems security threats and breaches |
||
|
|
● |
management of additional regulatory burdens |
||
|
|
● |
volatility in the market price for our Common Stock |
||
|
|
● |
potential imposition of additional sales practice requirements by the SEC |
||
|
|
● |
no dividends for the foreseeable future |
||
|
|
● |
future sales of Common Stock by existing shareholders causing the market price for our Common Stock to fall |
||
|
|
● |
the issuance of Common Stock in the future causing dilution |
||
If any of these risks or uncertainties materialize, or if assumptions underlying the forward-looking information prove to be incorrect, actual results might vary materially from those anticipated in the forward-looking information.
You should not rely upon forward-looking statements as predictions of future events. In addition, neither we nor any other person assumes responsibility for the accuracy and completeness of any of these forward-looking statements. The forward-looking statements contained in this Prospectus are made as of the date hereof, and we assume no obligation to update or supplement any forward-looking statements.
Please read “Risk Factors” herein and in other filings we make with the SEC for a more complete discussion of the risks and uncertainties mentioned above and for a discussion of other risks and uncertainties. All forward-looking statements attributable to us are expressly qualified in their entirety by these cautionary statements as well as others made in this annual report, and hereafter in our other SEC filings and public communications. You should evaluate all forward-looking statements made by us in the context of these risks and uncertainties. Note that forward-looking statements speak only as of the date of this annual report. Except as required by applicable law, we do not undertake any obligation to publicly correct or update any forward-looking statement.
AVAILABLE INFORMATION
We file certain reports under the Securities Exchange Act of 1934 (the “Exchange Act”). Such filings include annual and quarterly reports. The reports we file with the SEC are available on the SEC’s website (http://www.sec.gov).
PART I
Background
We were originally incorporated as Adelt Design, Inc. on March 31, 2011 to manufacture and market carpet binding art. Production and marketing of carpet binding art never commenced. After CLS Labs, Inc. (“CLS Labs”) acquired 55.6% of the outstanding shares of Common Stock of the Company, Jeffrey Binder, the Chairman, President and Chief Executive Officer of CLS Labs, was appointed Chairman, President and Chief Executive Officer of the Company. Subsequently, the Company adopted amended and restated articles of incorporation, thereby changing its name to CLS Holdings USA, Inc.
The Merger
On April 29, 2015, the Company entered into a merger agreement with CLS Labs and a newly-formed, wholly owned subsidiary of the Company (the “Merger Sub”) and effected the Merger (the “Merger”). Upon the consummation of the Merger, the separate existence of the Merger Sub ceased and CLS Labs, the surviving corporation in the Merger, became a wholly owned subsidiary of the Company, with the Company acquiring the stock of CLS Labs, abandoning its previous business, and adopting the existing business plan and operations of CLS Labs. CLS Labs is a company that plans to generate revenues through licensing, fee-for-service and joint venture arrangements related to its patented proprietary method of extracting cannabinoids from cannabis plants and converting the resulting cannabinoid extracts into saleable concentrates.
Historical Operations
Since 2014, one of the founders of CLS Labs has been developing a proprietary method of extracting cannabinoids from cannabis plants and converting the resulting cannabinoid extracts into concentrates such as oils, waxes, edibles and shatter. These concentrates may be ingested in a number of ways, including through vaporization via e-cigarettes, and used for a variety of pharmaceutical and other purposes. Internal testing of the cannabinoids extracted through our patent-pending proprietary process versus the cannabinoids resulting from the processes commonly used in the industry, the results of which were reviewed and confirmed by an independent laboratory, has revealed that our process produces a cleaner, higher quality product and a significantly higher yield than the cannabinoid extraction processes currently existing in the marketplace.
As CLS Labs was unable to obtain a license in Colorado to operate a cannabis processing facility due to residency requirements, on April 17, 2015, CLS Labs took its first step toward commercializing its then patent pending proprietary methods and processes by entering into an arrangement, as described in the section entitled “The Colorado Arrangement” below (the “Colorado Arrangement”. During 2017, we suspended our plans to proceed with the Colorado Arrangement due to regulatory delays and have not yet determined when we will pursue it again.
On April 24, 2018, we were issued a U.S. patent with respect to our proprietary method of extracting cannabinoids from cannabis plants and converting the resulting cannabinoid extracts into concentrates such as oils, waxes, edibles and shatter. These concentrates may be ingested in a number of ways, including through vaporization via electronic cigarettes, and used for a variety of pharmaceutical and other purposes. Internal testing of this extraction method and conversion process has revealed that it produces a cleaner, higher quality product and a significantly higher yield than the cannabinoid extraction processes currently existing in the marketplace. We have not commercialized our proprietary process. We plan to generate revenues through licensing, fee-for-service and joint venture arrangements related to our proprietary method of extracting cannabinoids from cannabis plants and converting the resulting cannabinoid extracts into saleable concentrates.
We intend to monetize this extraction method and generate revenues through (i) the licensing of our proprietary methods and processes to others, as in the Colorado Arrangement, (ii) the processing of cannabis for others, and (iii) the purchase of cannabis and the processing and sale of cannabis-related products. We plan to accomplish this through the acquisition of companies, the creation of joint ventures, through licensing agreements, and through fee-for-service arrangements with growers and dispensaries of cannabis products. We believe that we can establish a position as one of the premier cannabinoid extraction and processing companies in the industry. Assuming we do so, we then intend to explore the creation of our own brand of concentrates for consumer use, which we would sell wholesale to cannabis dispensaries. We believe that we can create a “gold standard” national brand by standardizing the testing, compliance and labeling of our products in an industry currently comprised of small, local businesses with erratic and unreliable product quality, testing practices and labeling. We also plan to offer consulting services through Cannabis Life Sciences Consulting, LLC (“CLS Consulting”), which will generate revenue by providing consulting services to cannabis-related businesses, including growers, dispensaries and laboratories, and driving business to our processing facilities.
The Colorado Arrangement
Licensing Agreement
On April 17, 2015, CLS Labs Colorado entered into a Licensing Agreement with Picture Rock Holdings, LLC (“PRH”) whereby, in exchange for a license fee payable over the ten (10) year term of the agreement, CLS Labs Colorado granted to PRH an exclusive license for the State of Colorado of certain proprietary inventions and formulas relating to the extraction from, separation and processing of marijuana to produce certain marijuana-infused products, including edibles, e-liquids, waxes and shatter, and to practice and use such extraction processes in conjunction with the manufacture, production, sale, and distribution of such Products.
Lease and Sublease
In connection with the Colorado Arrangement, on April 17, 2015, pursuant to an Industrial Lease Agreement, CLS Labs Colorado leased 14,392 square feet of warehouse and office space in a building in Denver, Colorado where certain intended activities, including growing, extraction, conversion, assembly and packaging of cannabis and other plant materials, are permitted by and in compliance with state, city and local laws, rules, ordinances and regulations. The Lease had an initial term of seventy-two (72) months and provided CLS Labs Colorado with certain renewal options. In August 2017, as a result of our decision to suspend our proposed operations in Colorado, CLS Labs Colorado asked its landlord to be relieved from its obligations under the Lease, but the parties have not yet reached an agreement on how to proceed.
Contemporaneously with the execution of the Lease, CLS Labs Colorado entered into a Sublease Agreement with PRH, thereby subletting the entire leased premises to PRH. As a result of our decision to suspend our plans to enter the Colorado market, PRH has vacated the subleased premises but the sublease remains effective.
Equipment Lease
In addition to the above-referenced Sublease, on April 17, 2015, CLS Labs Colorado and PRH entered into an Equipment Lease Agreement (the “PRH Equipment Lease”) whereby, in exchange for a lease payment, CLS Labs Colorado agreed to commence building a fully equipped lab at the leased premises, including purchasing all equipment necessary to extract, convert and provide quality control of all cannabis products of PRH. The term of the PRH Equipment Lease was to commences upon delivery of the equipment and terminate upon the earlier of ten (10) years from its effective date or such earlier date upon which the real property lease is terminated. Due to our suspension of plans to enter the Colorado market, the PRH Equipment Lease never commenced.
The Promissory Note
On April 17, 2015, CLS Labs Colorado loaned Five Hundred Thousand Dollars ($500,000) to PRH pursuant to a promissory note (the “Note”) to be used by PRH in connection with the financing of the building out, equipping, and development of the grow facility by PRH that will be operated by the Grower. Pursuant to the Note, as amended by the parties effective June 30, 2015, October 31, 2015, April 11, 2016 and May 31, 2016, PRH will repay the principal due under the Note in twenty (20) equal quarterly installments of Twenty Five Thousand Dollars ($25,000) commencing in the month following the month in which PRH commences generating revenue at the grow facility, which commencement is currently unknown, and continuing until paid in full. Interest will accrue on the unpaid principal balance of the Note at the rate of twelve percent (12%) per annum and will be paid quarterly in arrears commencing after such initial payment and continuing until paid in full. All outstanding principal and any accumulated unpaid interest due under the Note is due and payable on the five-year anniversary of the initial payment thereunder. Due to the suspension of our plans to enter the Colorado market, we cannot predict when or if the Note will be paid although PRH did make one payment under the Note during the fiscal year ended May 31, 2018.
Acquisition of Alternative Solutions
On June 27, 2018, the Company completed the purchase of all of the membership interests in Alternative Solutions and the Oasis LLCs from the members of such entities (other than Alternative Solutions). The closing occurred pursuant to a Membership Interest Purchase Agreement (the “Acquisition Agreement”) entered into between the Company and Alternative Solutions on December 4, 2017, as amended. Pursuant to the Acquisition Agreement, the Company initially contemplated acquiring all of the membership interests in the Oasis LLCs from Alternative Solutions. Just prior to closing, the parties agreed that the Company would instead acquire all of the membership interests in Alternative Solutions, the parent of the Oasis LLCs, from its members, and the membership interests in the Oasis LLCs owned by members other than Alternative Solutions. The revised structure of the transaction is referenced in the Oasis Note (as defined below), which modified the Acquisition Agreement.
Pursuant to the Acquisition Agreement, the Company paid a non-refundable deposit of $250,000 upon signing, which was followed by an additional payment of $1,800,000 paid in February 2018, for an initial 10% of each of the Oasis LLCs. At that time, the Company applied for regulatory approval to own an interest in the Oasis LLCs, which approval was received on June 21, 2018. On June 27, 2018, the Company made the payments to indirectly acquire the remaining 90% of the Oasis LLCs, which were equal to cash in the amount of $6,200,000 (less offsets for assumed liabilities), a $4.0 million promissory note due in December 2019 (the “Oasis Note”), and 22,058,823 shares of Common Stock. We used the proceeds of the Canaccord Special Warrant Offering to fund the cash portion of the closing consideration. On December 12, 2018, we were approved for the transfer of the remaining 90% interest.
The number of purchase price shares was equal to 80% of the offering price of the Company’s Common Stock in its last equity offering, which price was $0.34 per share. The Oasis Note is secured by a first priority security interest over the membership interests in Alternative Solutions and the Oasis LLCs, as well as by the assets of the Oasis LLCs. The Oasis Note bears interest at the rate of 6% per annum and both principal and accrued interest are due and payable in full on December 4, 2019 but may be prepaid at any time without penalty. We also delivered a confession of judgment to a third party neutral representative of the parties that will become effective, in general, if we default under the Oasis Note.
At the time of closing of the Acquisition Agreement, Alternative Solutions owed certain amounts to a consultant known as 4Front Advisors, LLC (“4Front”). In August 2019, we made a payment to this company to settle this dispute and the Oasis Note was reduced accordingly.
The sellers of the membership interests in Alternative Solutions are also entitled to a $1,000,000 payment from the Company on May 30, 2020 if the Oasis LLCs have maintained an average revenue of $20,000 per day during the 2019 calendar year. This amount was fully accrued at May 31, 2019.
None of the sellers of the membership interests in Alternative Solutions or the Oasis LLCs was affiliated with the Company prior to the closing. In connection with the closing, however, the Company employed Mr. Ben Sillitoe, the CEO and a member of Alternative Solutions, as the Chief Executive Officer of CLS Nevada, Inc., and Don Decatur, the COO of the Oasis LLCs, as the Chief Operating Officer of CLS Nevada, Inc.
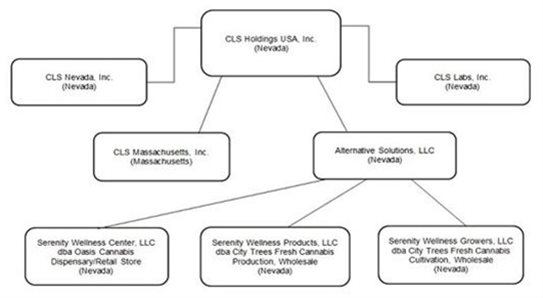
Corporate Structure
We have four direct and three indirect, active, wholly-owned subsidiaries, CLS Labs, CLS Nevada, Inc., CLS Massachusetts, Inc. and Alternative Solutions are owned directly, and Alternative Solutions owns 100% of the issued and outstanding membership interests of: (i) Serenity Wellness Center, LLC dba Oasis Cannabis Dispensary Retail Store (“Oasis”); (ii) Serenity Wellness Products, LLC dba City Trees Fresh Cannabis Production, Wholesale (“City Trees Production”); and (iii) Serenity Wellness Growers, LLC dba City Trees Fresh Cannabis Cultivation, Wholesale (“City Trees Cultivation”, together with City Trees Production, “City Trees” and together with Oasis and City Trees Production, the “Oasis LLCs”). The following diagram illustrates the inter-corporate relationships of the Company, and all of the parents own 100% of the issued and outstanding shares of their subsidiaries:

Notes:
|
|
(1) |
We own 100% of Alternative Solutions, CLS Nevada, Inc., CLS Labs, Inc., and CLS Massachusetts, Inc. |
|
|
(2) |
Alternative Solutions owns 100% of Oasis, City Trees Production and City Trees Cultivation. |
|
|
(3) |
All entities in the corporate chart were incorporated and are existing under the laws of the state of Nevada, except for CLS Massachusetts, Inc., which is a Massachusetts corporation. |
Nevada Operations
We own 100% of Alternative Solutions, which is a Nevada-based holding company that owns three separate entities with licenses to operate cannabis businesses within the State of Nevada. Oasis currently operates a retail marijuana dispensary within walking distance to the Las Vegas Strip. Its other subsidiaries, which do business as City Trees Cultivation and City Trees Production, currently operate a small-scale cultivation and product manufacturing facility, as well as a wholesale distribution operation in North Las Vegas. Management expects that the vertically integrated business model will drive strong margins to the bottom line on a large portion of existing sales at the dispensary as the build out of the Warehouse Facility becomes operational. (See section entitled “Expansion of Cultivation and Manufacturing Facilities” below).
Oasis’ retail dispensary is a single location operation in Nevada and occupies over 5,000 square feet of an over 20,000 square foot building This location, which is easily accessible by tourists, is currently open 24 hours per day for walk-in customers and in-store pickup. It also delivers cannabis to residents between the hours of 9:00 AM and 8:00 PM. The central location provides logistical convenience for delivery to all parts of the Las Vegas valley.
City Trees’ wholesale operations, which occupies approximately 1,150 square feet of a 22,000 square foot warehouse (the “Warehouse Facility”), began sales to third parties in August 2017. It had made sales to over 45 external customers by May 31, 2019. Its existing product line includes vaporizers, tinctures, capsules, and concentrates. At present, the City Trees cultivation facility only grows breeding stock to preserve valuable genetics and does not offer its crops for sale or processing. As a result, all raw materials for manufacturing are sourced from third parties.
Market Growth
According to the 2017 report compiled by ArcView Market Research, legal cannabis sales in the U.S. grew by over 37% in 2017 to $9.5 billion. This growth trend is expected to continue as more states legalize medical and retail cannabis and as more consumers choose to make legal cannabis purchases instead of buying through traditional sources. Consumers who are learning about new research supporting the health and the perceived medical benefits of cannabis will be a secondary source of strong growth in the market for the next several years.
Cannabis sales in Nevada have exceeded all expectations since recreational sales began on July 1, 2017. The Nevada Department of Taxation (“NV DOT”) indicated it had exceeded its marijuana tax collection projection for the entire 2018 fiscal year after only nine months of sales.1 The NV DOT Marijuana Revenue Statistics for Taxable Sales Reported by Adult-use Retail Stores and Medical Dispensaries for fiscal year 2019 show a 20% market growth over fiscal year 2018 through May 31, 2019 in both periods.2 Management believes that the Nevada market will continue to grow at double digit rates for the next few years. This expectation is supported by sales trends in other legal markets like Colorado and Washington.
Internal Growth Strategy
Oasis expects to continue to grow its dispensary market share both organically and by adding additional locations within the Nevada market. Oasis will seek to expand its footprint throughout the state in select locations with access to tourists or in residential areas with above average median income. The locations of the potential acquisitions will only matter to the extent that they are in preferable local jurisdictions. For licensing purposes, the physical location of a marijuana establishment in Nevada may be moved if it remains in the same local municipality or jurisdiction.
City Trees’ wholesale growth strategy focuses on completion of Phase 1 of the planned build out of the Warehouse Facility, with secondary focus on adding new customers and increasing product line penetration at each customer’s retail location. City Trees has about 40 customers with regular recurring orders at dispensaries located throughout Nevada. Oasis currently purchases about $30,000 per month in products from City Trees, which represents only about 10% – 15% of the total retail sales. When City Trees is able to grow and release its wholesale cannabis flower, Oasis will be able to purchase about $100,000 worth of product as it replaces some of its current third party vendors with City Trees. It is expected that other existing wholesale customers will also replace some of their current suppliers’ flower with City Trees once it has become available. At present, City Trees competes with companies that grow their own raw materials. Because City Trees currently purchases raw materials from third parties, and because competition prevents City Trees from pricing its product in a manner that would generate a typical gross margin, at present, City Trees is operating with lower gross margins than it will achieve after the completion of the Warehouse Facility expansion, which is expected in the fourth quarter of calendar year 2019. Oasis expects gross margins and overall cash flow to improve as the build out of the Warehouse Facility becomes operational. (See section entitled “Expansion of Cultivation and Manufacturing Facilities” below.)
1 State of Nevada Department of Taxation “April Marijuana Revenue Statistics News Release”. June 28, 2018, accessed July 3, 2018. Available at: https://tax.nv.gov/uploadedFiles/taxnvgov/Content/TaxLibrary/News-Release-April-Marijuana.pdf.
2 State of Nevada Department of Taxation “Marijuana Tax Revenue – Fiscal Year 2019”. Excel Spreadsheet accessed at https://tax.nv.gov/Publications/Marijuana_Statistics_and_Reports/
Dispensary Operations
Oasis opened as a medical cannabis dispensary in 2015 and began retail sales to adults over the age of 21 on July 1, 2017. Customers and patients can browse the selection of inventory on display and ask questions to qualified staff with minimal wait times. The dispensary was recently renovated and a “grand re-opening” was held in early April 2019. The renovations included new building signage that increased visibility along Industrial Road, an updated sales floor with improved product displays that enhanced customer interactions.
Inventory Management
All inventory is tracked in the state-mandated METRC seed to sale tracking system. Additionally, we have implemented Treez for our point of sale and internal inventory management system. Each item is stored in a designated physical location that is also reflected in the inventory control system. All products are prepackaged before arriving at the retail store and a barcode is added to each package to ensure the proper products are fulfilled in each order. Treez synchronizes its sales and inventory data with METRC, but we also regularly reconcile the two systems for additional assurance of compliance with state mandated inventory tracking accuracy. Regular, independent inventory counts ensure that any physical variances from the tracking system are detected and addressed immediately. All product that is unusable is destroyed and logged with photo-evidence according to state regulations.
Product Selection
Product selections are currently managed by a team comprised of the Retail Operations Manager, Assistant General Manager, and Inventory Team Leader. As Oasis adds new locations, it will form a centralized purchasing team that will ensure there is consistent product selection across all locations. The Retail Operations Manager is responsible for negotiating bulk purchase discounts and maintaining target gross margins. The Retail Operations Manager is also responsible for quality assurance and product mix. Each new vendor is researched, and their operations are visited whenever possible. Product samples are distributed to various employees and feedback is reviewed before making final product decisions. Oasis carries between 30 and 40 different cultivars or “strains” of cannabis flowers in addition to a wide variety of cannabis products such as vaporizers, concentrated oil, edibles, capsules, tinctures, and beverages.
Payment System and Banking
Payments made at Oasis are currently completed with cash only. Cash risk is minimized by making regular deposits in our bank account at a credit union. Cash deposits are picked up by an armored truck and taken to the local Federal Reserve Bank where the deposit is made on our behalf.
Home Delivery and In-Store Pickup
Home delivery is currently about 15% of the total sales mix of Oasis. Customers can call or place orders online for both pickup and delivery. There is currently no fee for delivery but there are minimum order amounts based on the distance from the store. Home deliveries average well over $100 per order, which is about 75% higher than in-store orders. Oasis is centrally located within the Las Vegas valley which makes it roughly equally distant from all areas of town. This allows the store to have a much wider geographic reach than it otherwise would. Many locals work on the Las Vegas Strip close to the store and will shop there when going to and from a shift. Offering delivery also allows them to conveniently make a purchase from Oasis without having to drive past a cannabis store that might be located closer to their homes. Many consumers prefer the convenience of home delivery and this allows Oasis to be their dispensary of choice regardless of how close they live to the store.
Pricing Strategy
Oasis targets at least a 53% gross margin when determining pricing for any given product. Market dynamics such as supply, demand, and competitive pressure can cause variances from the target. The assistant general manager of Oasis, as part of the purchasing team, conducts a pricing survey for all new products to determine which of the competition in close proximity carries the product and how much such competition is charging for similar products. Oasis offers a price match guarantee to minimize the risk of losing customers to competitors’ daily specials or discounts, and also sets prices to be consistent with the selection of product that is offered by competitor dispensaries in the area.
Marketing Strategy
Oasis uses a variety of methods to reach consumers including billboards, paid digital static and video online ads, social media, marketing to rideshare drivers, and social engagement through a calendar of events at its community center called Community Oasis. It has recently begun using radio advertisement to gain extra exposure for special events, such as the “grand-reopening” and April 20th celebrations in 2019. These radio advertisements have proven to be effective and cost efficient only when there is a new event or great offer to share, so they are used only for a limited time and when there is a compelling message. Oasis employs a Director of Marketing who is responsible for developing and implementing the quarterly marketing strategies that coincide with different seasons and events in Las Vegas.
Cultivation, Production & Wholesale Sales Operations
City Trees’ wholesale operations primarily consists of purchasing finished distilled cannabis oil from third party vendors and formulating it into a variety of finished products for sales and distribution to retail cannabis stores and medical dispensaries throughout Nevada. Although City Trees has the capability to conduct extraction, conversion and processing activities, it does not presently conduct many of these activities because it is not manufacturing its own raw materials. In the future, City Trees plans to conduct these activities using both its internally developed methods as well as our patented process. (See section entitled “Expansion of Cultivation and Manufacturing Facilities” below.)
Due to the small size of the existing Oasis grow operation, it currently only cultivates plants for breeding and to preserve quality stock and does not harvest its plants for either production or for sale to third parties.
Expansion of Cultivation and Manufacturing Facilities
City Trees Cultivation is in the preliminary stages of expanding its grow operation and implementing additional manufacturing operations using both Alternative Solutions’ existing processing methods and our patented processing methods. City Trees Cultivation intends to build out a processing facility and a grow operation to manufacture product for Oasis. As part of this expansion, City Trees Cultivation intends to construct a multi-level grow operation in the Warehouse Facility.
During the next twelve months we expect to complete phase 1 (“Phase 1”) of our expansion plan (the “Expansion Plan”). The timeline for the commencement and completion of phase 2 of the Expansion Plan (“Phase 2”) is currently not known. We do not require additional financing to complete Phase 1 of the Expansion Plan.
Phase 1 construction is currently underway. It will add 2,500 square feet of cannabis extraction and product manufacturing operations within the current facility. The cultivation portion of the expansion will add one room for mother plants and two rooms for flowering stage in addition to rooms for trimming, curing, drying, packaging, and storage. We expect the power upgrade aspect of Phase 1 to take six months and Phase 1 to be completed within the next 12 months. Phase 2 includes the possible addition of 7 additional flower rooms to the remaining unused portion of the building. We anticipate that City Trees Cultivation will use state of the art LED grow lights and a vertical racking system to dramatically reduce energy costs and increase growth capacity.
The anticipated steps for Phase 1 are as follows:
|
1. |
Finalize construction plan revisions and execute a construction contract for Phase 1 cultivation. The contract for Phase 1 production has been executed and construction is currently underway. The Phase 1 cultivation plans are in the final stages and a construction contract is expected to signed within 60 days. |
|
2. |
Floor plans and operational plans including standard operating procedures and required equipment have been submitted for approval to the NV DOT. |
|
3. |
Building permits have been obtained from North Las Vegas Building Department for Phase 1 production. Building permits will be obtained from North Las Vegas Building Department for Phase 1 cultivation. |
|
4. |
Power upgrades will be completed. |
|
5. |
Tenant improvements for Phase 1 will be completed. |
The anticipated timing and steps for Phase 2 are to be determined at a later date.
The Company currently anticipates completion of Phase 1 in the fourth quarter of the 2019 calendar year and the launch of the new production facilities in the first calendar quarter of 2020.
The Warehouse Facility also has a 34,000 square foot enclosed yard that City Trees Cultivation may develop into a greenhouse (the “Greenhouse Expansion”) in the future as doing so would further reduce raw materials and manufacturing costs by using mostly sunlight instead of electricity. The Greenhouse Expansion is separate from the Phase 1 and Phase 2 expansions and will be completed on the existing, enclosed asphalt yard after the Warehouse Facility is operating at full capacity within the current structure, i.e. Phase 1 and Phase 2 are completed.
As City Trees Cultivation completes the phases of the Expansion Plan, we expect to capture additional margin as less of the raw materials will be purchased from third parties. City Trees also plans to introduce a new revenue stream from contract manufacturing fees earned from strong brands/companies that do not own licensed production facilities in Nevada.
Product Line
City Trees offers the following product lines to its wholesale customers:
● The vaporizer and concentrate product line consists of proprietary blends of cannabis oil and terpenes filled into custom branded City Trees vaporizers that utilize ceramic heating technology to deliver clean, even heat without using a wick like most traditional vaporizers.
● The City Trees product line of capsules is known as City Caps and includes CBD and THC blends in ratios of 10 to 1, 4 to 1, and 1 to 4. The blends are named cannabidiol (“CBD”), Rise, and Rest, respectively
● The City Trees line of tinctures includes a 20 to 1, 10 to 1, and a 1 to 1 CBD to THC ratio as well as a THC only version. The tinctures are available in 3 different carriers and flavors, MCT oil, agave nectar, and chocolate agave nectar.
Pricing Strategy
The raw materials cost inputs have dropped over the last year because of an increase in the supply of raw distillate oil. The cost is expected to drop even further once we are producing our own raw distillate oil. We target retail prices to be competitive against other high-end brands and to deliver strong margins to City Trees and its retail customers.
Vertical Farming
As wholesale cannabis flower and trim moves toward becoming priced like a commodity, minimizing output costs will become more important than ever before. Wholesale price compression will reduce profitability and put many operators who are not able to grow outdoors or in greenhouses in difficult positions. Vertical farms use cubic feet instead of square feet to calculate how much space is available for cultivation. Phase 1 and Phase 2 construction project plans for 20-foot ceilings that can accommodate up to 3 tiers of grow canopy, essentially tripling the potential output in the building. Management expects City Trees will start with 2 tiers in most areas during phase 1 of its expansion and test 3 tiers on a smaller scale before rolling it out across the entire facility in phase 2.
The vertical farm will reduce electricity and rent costs per pound but has the potential to increase labor costs per pound if proper automation is not used. City Trees plans to utilize a moderate amount of automated technology to offset the potential additional labor costs. Automated watering, feeding, lighting systems are in the design phase.
Energy Efficient Heating & Cooling
In addition to using LED lights to conserve energy, City Trees plans to utilize natural gas heat pumps to minimize its heavy reliance on electricity. The units are able to heat and cool critical areas of the building using natural gas instead of relying on the already over-burdened electrical system of an indoor cultivation facility.
Single Stream Inventory
In Nevada, as long as a wholesale facility holds both a medical and a recreational license, it may sell products to dispensaries that may be sold to both recreational and medical customers. As long as the dispensary also holds both licenses, the inventory may be sold to either type of customer as long as it came from a wholesale company with both license types. This reduces logistical challenges that would otherwise arise from having two separate streams of inventory to service the medical and adult-use segments.
Licenses
A Retail Marijuana Store License or Medical Marijuana Dispensary Registration Certificate allows for the sale of cannabis products to the applicable end consumer. A company must hold both licenses to be able to sell products to both types of consumers. A retail marijuana store may also deliver to residents in Nevada without any additional licensing. Both local and state licenses are required.
A Retail (adult-use or recreational) Marijuana Cultivation or Medical Marijuana Cultivation Registration Certificate allows the holder to grow as much cannabis as it can in its approved production space. There is no limitation to the number of plants that maybe be grown at any time. The state only approves the production space regarding compliance, not size.
A Retail (adult-use or recreational) Marijuana Product Manufacturing license or Medical Marijuana Production Registration Certificate allows for the extraction, conversion, and manufacturing of raw cannabis material into finished consumer packaged goods. The NV DOT must approve all formulas, processes, equipment, products, and packaging prior to any manufacturing or sales.
A Retail (adult-use or recreational) Marijuana Distributor License allows licensees to deliver wholesale products from a cultivator or manufacturer to a retail store. This is only a requirement for products that could be sold to recreational customers. Many vertically integrated operators are forced to use third party distributors to deliver products from their wholesale facilities to their own stores and to other customers. City Trees holds one of only 29 distributor licenses that exist to serve the more than 60 dispensaries and 195 wholesalers in the State. Oasis is licensed to operate in the city of Las Vegas as a Dual Use Marijuana Business, and in the State of Nevada as a Medical Marijuana Dispensary Establishment and a Retail Marijuana Store. City Trees Production is licensed to operate in the state of Nevada as a Medical Marijuana Production Establishment, a Retail Marijuana Product Manufacturing facility and a Retail Marijuana Distributor. City Trees Production is also licensed to operate in the state of Nevada as a Medical Marijuana Cultivation Facility and a Retail Marijuana Cultivator. Please see “Our Business – Regulation and Licensure – Oasis LLC Licenses” for a complete list of state and local licenses held by the Oasis LLCs.
Specialized Skill & Knowledge
Commercial cannabis cultivation requires access to employees with specialized skills and knowledge in order to maximize harvest quality and yield in addition to having the capacity for developing new varieties. Botanical extraction of concentrated oils, product formulation and product manufacturing each require their own specific sets of specialized skill and knowledge to ensure maximization of yields and quality from extraction and to create consistent, high quality products. Additionally, the operation of a quality retail cannabis store requires extensive product knowledge to provide the optimal experience for customers. Each of these operations requires extensive knowledge and understanding of the Nevada regulatory landscape to ensure compliance with all local and state laws and regulations.
The COO of CLS Nevada, Inc. has gained important skills and knowledge through experience with all areas needed to run a successful cultivation operation. With these skills and knowledge, we expect the Company to continue to develop unique, new strains that are only available to City Trees and will build on the current knowledge of the organization through testing new techniques and technologies in a small research and development room within the cultivation facility. The previous experience of the management team of CLS Nevada, along with independent consultation, is the basis for Oasis’ proprietary standard operating procedures that we believe will ensure consistent quality and yield performance. The COO of CLS Nevada has practical experience with the extraction of cannabis including no-solvent, butane, carbon dioxide and the finishing of the extracts into consumer-packaged goods.
The extraction / product formulation team includes employees with hands on experience in cannabis extraction and product manufacturing in addition to employees with undergraduate chemistry degrees and limited experience in cannabis extraction. This provides access to both the technical and hands-on applications of knowledge that benefits product formulation in addition to extraction efficiency and productivity.
The leadership at CLS Nevada is knowledgeable in all the products available in the United States market because the leadership at Oasis has operated in Nevada since the beginning of medical cannabis sales.
We conduct ongoing training to ensure compliance with all laws and regulations. The leadership of each business unit attends regular compliance training conducted by local and state officials which provides content and updates for internal training.
In addition to our internal resources, there is a broad market of skilled employees with cannabis knowledge and experience in Nevada to facilitate growth of the labor force.
Competitive Conditions
We currently operate in the Nevada cannabis market, which has limited licensing opportunities for retail locations in accordance with state regulations. These conditions create significant barriers to entry for new competition.
There is currently no legal limitation on the number of cultivation and product manufacturing licenses that may be issued and there is no limitation on how much can be grown or produced with those licenses.
The limitation on the number of licenses available for retail creates a significant barrier to entry for potential competition in the retail cannabis market. Acquisition is the only method available for most companies to enter the state’s retail cannabis market absent changes in legislation. There is also a 10% legal limitation on the number of retail licenses that may be owned by any one entity within a given county. The size and number of locations in a potential acquisition are limited as a result. These conditions mitigate the risk of losing market share to new companies entering the Nevada retail market.
The wholesale market, however, is more fluid. At present, both supply and demand for raw cannabis are increasing, but the increase in supply precipitated by the commencement of recreational sales is outpacing the increase in demand. As a result, Nevada wholesale prices have decreased over the last year. We have undertaken and, in some cases, completed various expansion projects to meet the additional demand but we are carefully watching changes in the supply market. Most of the additional supply has been provided by existing participants within the market as very few new cultivation licenses have been issued. The ability to expand facilities without limitation will allow the market to reach an equilibrium wholesale price point without the need to license additional operators. Although there is no legal limitation on cultivation and production licenses, we do not currently anticipate that new licenses will be issued.
Regardless of whether supply remains high, we believe we can benefit from market conditions. A low cost for raw cannabis will likely benefit our production operation, which is expected to ramp up once the facility that will utilize our new and more efficient patented technology is operational, as we expect that we can produce more quality product with less raw cannabis, thus partially offsetting the impact of low wholesale prices. Low wholesale prices could also benefit our dispensary as this reduces our cost of product. If conditions change and supply is reduced, we can expand our cultivation facility, as presently planned.
Components
Raw materials for processing and manufacturing are available from a variety of sources. Oasis maintains relationships with various suppliers for each key component of the raw materials to mitigate vendor concentration risk. City Trees wholesale operations is the sole purchaser of raw materials within the organization because the retail operation only stocks finished consumer packaged products. All raw materials are currently purchased from third parties. City Trees is expected to be able to supply a large portion of the raw cannabis material upon completion of phase 1, but certain items will always come from third parties. The following table describes the key components of the supply chain for City Trees products:
|
Raw Material Item |
Description |
Sources |
# of Suppliers |
Pricing |
Internal Sourcing |
|
Raw Cannabis Trim |
Raw cannabis leaf that is trimmed from raw flowers that will be sold directly to consumers. Trim makes up the majority of what is extracted into oil. |
Nevada Licensed Cultivators (115 active licenses as of April 2018) |
5+ |
Wholesale prices are currently in the range of $500 - $750 per pound. Target pricing is $350 per pound in order to match the cost of sourcing finished bulk oil. |
Gradually increasing amount will be sourced internally upon completion of Phase 1 and Phase 2. |
|
Raw Cannabis Flower |
Raw cannabis flower is typically trimmed, packaged and sold to consumers or it is rolled into pre-rolled joints, packaged and sold to consumers. City Trees is currently not purchasing or harvesting flower. |
Nevada Licensed Cultivators (115 active licenses as of April 2018) |
5+ |
Wholesale prices currently range from $1,500 - $2,500 per pound. |
Gradually increasing amount will be sourced internally for City Trees upon completion of Phase 1 and Phase 2. |
|
Bulk Distillate Cannabis Oil
|
Cannabis oil refined through distillation processes that maximize potency and remove impurities. |
Nevada Licensed Product Manufacturers (80 active licenses as of April 2018) |
4+ |
Wholesale prices currently range from $10 - $14 per gram. |
Gradually increasing amount will be sourced and processed internally upon completion of Warehouse Expansion. |
|
Custom All-in-One Disposable Vaporizer Pens |
Cannabis oil vaporizer “pens” with ceramic heating that contain a single use battery charge customized with City Trees logos and imagery. |
Distributors of Chinese Manufacturing Products |
2 |
$3.35 each |
N/A |
|
Vaporizer Pen Cartridges and Custom Batteries |
Cannabis oil vaporizer cartridges with ceramic heating that attach to a rechargeable battery customized with City Trees logos and imagery. |
Distributors of Chinese Manufacturing Products |
2 |
Cartridges: $2.50 each Custom Batteries: $3.25 each |
N/A |
|
Vegan Capsules |
Empty capsules that are filled with proprietary blends of cannabis oil and terpenes |
Online Medical Supply Companies |
2 |
1.3 cents per capsule |
N/A |
|
Botanical Terpenes |
Natural compounds found in essential oils of plants with strong fragrance and flavor. Some terpenes have been shown to be biologically active with specific effects |
Domestic online suppliers of cannabis-derived and non-cannabis derived terpenes. |
2 |
Isolated Terpenes: $290 per kilogram |
Some terpenes will be sourced internally through a fractional distillation process. |
|
CBD Isolate |
Cannabidiol (CBD) in powder form that is 99.9% pure CBD |
Domestic Industrial Hemp Growers and Processors |
2 |
Wholesale prices range from $7,000 - $10,000 per kilogram |
N/A |
Intellectual Property
Domains
We have protected Internet domain names with the following registered domains as of the date of this Prospectus:
|
|
● |
https://www.clsholdingsinc.com/ |
|
|
● |
https://oasiscannabis.com/ |
|
|
● |
http://www.citytrees.com/ |
Patent and Trademarks
We have developed extraction and processing methods that are proprietary and, on April 24, 2018, the Company (via CLS Labs) was awarded a non-provisional U.S. utility patent for cannabidiol extraction and conversion process (the “Extraction Process”) by the United States Patent and Trademark Office (U.S. patent number 9,950,976 B1). The Extraction Process is expected to result in increased product consistency, cost savings for growers, and increased anticipated revenues for us due to the larger amount of Delta-9 THC that we believe it can produce. We expect to use a version of the patented technology on a smaller scale in connection with Phase 1 of the Expansion Plan.
Internal testing of the Extraction Process has revealed that such process produces a cleaner, higher quality product and a higher yield than the cannabinoid extraction processes currently existing in the marketplace. We have not commercialized the Extraction Process. We plan to generate revenues through licensing, fee-for-service and joint venture arrangements related to the Extraction Process from cannabis plants and converting the resulting cannabinoid extracts into saleable concentrates.
We intend to monetize the Extraction Process and generate revenues through (i) the licensing of its patented processes to others, (ii) the processing of cannabis for others, and (iii) the purchase of cannabis and the processing and sale of cannabis-related products. We plan to accomplish this through the acquisition of companies, the creation of joint ventures, through licensing agreements, and through fee-for-service arrangements with growers and dispensaries of cannabis products. We then intend to explore the creation of its own brand of concentrates for consumer use, which it would sell wholesale to cannabis dispensaries. We believe that it can standardize the testing, compliance and labeling of its products in the cannabis industry.
Employees
As of July 1, 2019, the Oasis LLCs had 75 employees. The employees are distributed among the following departments:
|
Nevada Market Administration |
|
Number of Employees |
|
Administrative Accounting |
|
3 3 |
|
Executive |
|
1 |
|
Oasis Cannabis Retail |
|
|
|
Product Sales and Customer Service Inventory Control Dispatch / Delivery Safety / Security Management / Leadership Communications / Marketing |
|
26 5 6 9 6 1 |
|
City Trees Wholesale |
|
|
|
Wholesale Sales and Distribution Leadership Cultivation / Product Manufacturing Inventory Control |
|
2 1 7 1 |
|
Total Employees |
|
71 |
We believe in equal opportunity employment and we recruit, hire and promote individuals that are best qualified for each position without regard to race, color, creed, sex, national origin or handicap. We pride ourselves on using a selection process that recruits people who are trainable, co-operative and share the core values of the Company. Our employees are highly-talented individuals who have educational achievements ranging from masters and undergraduate degrees in a wide range of disciplines, as well as staff who have been trained on the job to uphold the highest standards set as a Company.
We recruit based on a rigorous interview process to ensure the right candidates are selected for the Company and the individual team. In addition to adherence to our core values, it requires that each employee acts with integrity and constant striving to uphold the highest professional standards.
In addition, the safety of our employees is a priority and we are committed to the prevention of illness and injury through the provision and maintenance of a healthy workplace. We take all reasonable step to ensure staff are appropriately informed and trained to ensure the safety of themselves as well as others around them.
In addition to the Oasis employees, the Company employs three executive and management personnel and engages one consultant in a management capacity.
Growth Strategy
Our growth strategy includes the following plans:
|
|
● |
Securing capital for the construction of processing centers. |
|
|
● |
Obtaining the necessary state and local licensure for each proposed facility. |
|
|
● |
Securing initial licensing, processing or sales arrangements, as applicable, with growers and dispensaries. Such arrangements may result from marketing efforts, relationships within the industry or the CLS Consulting business. |
|
|
● |
Constructing processing facilities. |
|
|
● |
Expanding per-facility capacity and increasing revenues. |
|
|
● |
Developing a national brand of cannabis concentrates, which will be sold wholesale to dispensaries, through standardization of the testing, compliance and labeling process. |
We may also grow by acquiring existing cannabis industry companies that will benefit from the use of our proprietary technology as well as other companies in the cannabis industry that are compatible with our proposed operations.
Regulation and Licensure
Despite 33 states and the District of Columbia, Puerto Rico and Guam having legalized or decriminalized marijuana use for recreational or medical purposes, the prescription, use and possession of marijuana remains illegal under federal law. As such, although we will only operate processing facilities in states that permit the possession, sale and use of cannabis, certain activities of our business, including the possession of cannabis for processing and the sale of cannabis concentrates, will be in violation of federal law.
We, through the Oasis LLCs, are directly involved in the cultivation, distribution and sale of cannabis in the State of Nevada. All of our operations are in the United States. Therefore, our balance sheet and operating statement exposure to U.S. marijuana-related activities is 100%.
Enforcement of United States Federal Laws
In the United States, cannabis is highly regulated at the state level. To our knowledge, over half of the United States of America, plus the District of Columbia, Puerto Rico and Guam have legalized cannabis in some form. California, Nevada, Massachusetts, Maine, Washington, Oregon, Colorado, Vermont, Alaska, Michigan, and the District of Columbia have legalized the recreational use of cannabis. Maine and Michigan have yet to begin their recreational cannabis commercial operations. Illinois will be the eleventh (11th) State to introduce a legal cannabis market launching sales on January 1, 2020. Fourteen additional states have legalized CBD, low Tetrahydrocannabinol (THC) oils for a limited class of patients. Notwithstanding the permissive regulatory environment of cannabis at the state level, cannabis continues to be categorized as a Schedule I controlled substance under the Controlled Substances Act (codified in 21 U.S.C.A. Section 812). Under United States federal law, a Schedule I drug is considered to have a high potential for abuse, no accepted medical use in the United States, and a lack of accepted safety for the use of the substance under medical supervision. Federal law prohibits commercial production and sale of all Schedule I controlled substances, and as such, cannabis-related activities, including without limitation, the importation, cultivation, manufacture, distribution, sale and possession of cannabis that remain illegal under U.S. federal law. It is also illegal to aid or abet such activities or to conspire or attempt to engage in such activities. Strict compliance with state and local laws with respect to cannabis may neither absolve the Company of liability under U.S. federal law, nor provide a defense to any federal proceeding brought against the Company. An investor’s contribution to and involvement in such activities may result in federal civil and/or criminal prosecution, including, but not limited to, forfeiture of his, her or its entire investment, fines and/or imprisonments.
As a result of the conflicting views between states and the federal government regarding cannabis, investments in, and the operations of, cannabis businesses in the U.S. are subject to inconsistent laws and regulations. The so-called “Cole Memorandum” or “Cole Memo” issued by former Deputy Attorney General James Cole on August 29, 2013 and other Obama-era cannabis policy guidance, discussed below, provided the framework for managing the tension between federal and state cannabis laws. Subsequently, as discussed below, Attorney General Jeff Sessions rescinded the Cole Memo and related policy guidance. Although no longer in effect, these policies, and the enforcement priorities established within, appear to continue to be followed during the Trump administration and remain critical factors that inform the past and future trend of state-based legalization.
On January 4, 2018, former Attorney General Jeff Sessions rescinded the Cole Memo, the Cole Banking Memorandum, and all other related Obama-era DOJ cannabis enforcement guidance. While the rescission did not change federal law, as the Cole Memo and other DOJ guidance documents were not themselves laws, the rescission removed the DOJ’s formal policy that state-regulated cannabis businesses in compliance with the Cole Memo guidelines should not be a prosecutorial priority. Notably, Attorney General Sessions’ rescission of the Cole Memo has not affected the status of the U.S. Department of the Treasury’s Financial Crimes Enforcement Network (“FinCEN”) memorandum issued by the Department of Treasury, which remains in effect. This memorandum outlines Bank Secrecy Act-compliant pathways for financial institutions to service state-sanctioned cannabis businesses, which echoed the enforcement priorities outlined in the Cole Memo. In addition to his rescission of the Cole Memo, Attorney General Sessions issued a one-page memorandum known as the “Sessions Memorandum”. The Sessions Memorandum explains the DOJ’s rationale for rescinding all past DOJ cannabis enforcement guidance, claiming that Obama-era enforcement policies are “unnecessary” due to existing general enforcement guidance adopted in the 1980s, in chapter 9.27.230 of the USAM. The USAM enforcement priorities, like those of the Cole Memo, are based on the use of the federal government’s limited resources and include “law enforcement priorities set by the Attorney General,” the “seriousness” of the alleged crimes, the “deterrent effect of criminal prosecution,” and “the cumulative impact of particular crimes on the community.” Although the Sessions Memorandum emphasizes that cannabis is a federally illegal Schedule I controlled substance, it does not otherwise instruct U.S. Attorneys to consider the prosecution of cannabis-related offenses a DOJ priority, and in practice, most U.S. Attorneys have not changed their prosecutorial approach to date. However, due to the lack of specific direction in the Sessions Memorandum as to the priority federal prosecutors should ascribe to such cannabis activities, there can be no assurance that the federal government will not seek to prosecute cases involving cannabis businesses that are otherwise compliant with state law.
Such potential proceedings could involve significant restrictions being imposed upon the Company or third parties, and also divert the attention of key executives. Such proceedings could have a material adverse effect on our business, revenues, operating results and financial condition as well as our reputation, even if such proceedings were concluded successfully in favor of the Company. See “Risk Factors”.
For the reasons set forth above, our existing operations in the United States, and any future operations or investments the Company may engage in, may become the subject of heightened scrutiny by regulators, stock exchanges and other authorities in Canada. As a result, the Company may be subject to significant direct and indirect interaction with public officials. There can be no assurance that this heightened scrutiny will not in turn lead to the imposition of certain restrictions on our ability to operate in the United States or any other jurisdiction. See “Risk Factors”.
Government policy changes or public opinion may also result in a significant influence over the regulation of the cannabis industry in the United States or elsewhere. A negative shift in the public’s perception of medical cannabis in the United States or any other applicable jurisdiction could affect future legislation or regulation. Among other things, such a shift could cause state jurisdictions to abandon initiatives or proposals to legalize medical cannabis, thereby limiting the number of new state jurisdictions into which the Company could expand. Any inability to fully implement our expansion strategy may have a material adverse effect on our business, financial condition and results of operations. See “Risk Factors”.
Further, violations of any federal laws and regulations could result in significant fines, penalties, administrative sanctions, convictions or settlements arising from civil proceedings conducted by either the federal government or private citizens, or criminal charges, including, but not limited to, disgorgement of profits, cessation of business activities or divestiture. This could have a material adverse effect on the Company, including its reputation and ability to conduct business, its holding (directly or indirectly) of medical cannabis licenses in the United States, the listing of its securities on various stock exchanges, its financial position, operating results, profitability or liquidity or the market price of its publicly traded shares. In addition, it is difficult for the Company to estimate the time or resources that would be needed for the investigation of any such matters or its final resolution because, in part, the time and resources that may be needed are dependent on the nature and extent of any information requested by the applicable authorities involved, and such time or resources could be substantial. See “Risk Factors”.
United States Enforcement Proceedings
An appropriations rider contained in the fiscal year 2015, 2016, 2017, 2018 and 2019 Consolidated Appropriations Acts (formerly known as the “Rohrabacher-Farr Amendment”; now known as the “Rohrabacher-Blumenauer Amendment” and currently proposed for the next appropriations rider as the “Joyce Amendment”, referred to herein as the “Amendment”) provides budgetary constraints on the federal government’s ability to interfere with the implementation of state-based medical cannabis laws. The Ninth Circuit Court of Appeals and other courts have interpreted the language to mean that the DOJ cannot expend funds to prosecute state-law-abiding medical cannabis operators complying strictly with state medical cannabis laws. The Amendment prohibits the federal government from using congressionally appropriated funds to prevent states from implementing their own medical cannabis laws. Previously the Amendment was extended until December 8, 2018, as part of the passage of an emergency aid package. In February 2019, President Trump renewed the Rohrabacher Amendment as part of the spending bill and it shall remain valid through September 30, 2019. Through his signing statement, President Trump reiterates that the Department of Justice may not use any funds to prevent implementation of medical marijuana laws by various States and territories, and “I will treat this provision consistent with the President’s constitutional responsibility to faithfully execute the laws of the United States.” Continued reauthorization of the Amendment is predicated on future political developments and cannot be guaranteed. If the Amendment expires, federal prosecutors could prosecute even state-compliant medical cannabis operators for conduct within the five-year statute of limitations. The Amendment does not protect state legal adult-use cannabis businesses and the DOJ may spend funds to prosecute persons that are operating in accordance with state adult use cannabis laws. However, the United States Congress recently passed the Blumenaur-McClintock-Norton Amendment which would provide legal protection for all state legal cannabis activities. It is unclear whether the amendment language will be included in the Senate appropriations language. In addition to the amendment, three separate proposed pieces of legislation have been introduced by members of Congress that would legalize marijuana at a federal level, although it is uncertain if any of the proposed bills will gain any traction.
Ability to Access Public and Private Capital
We have historically, and continue to have, access to equity and debt financing from the public and prospectus exempt (private placement) markets in Canada and, to a lesser extent, in the United States. Our executive team and board of directors also have extensive relationships with sources of private capital (such as funds and high net worth individuals), that could be investigated at a higher cost of capital. If such equity and/or debt financing was no longer available in the public markets due to changes in applicable law, then the Company expects that it would have access to raise equity and/or debt financing privately.
Although we are not able to obtain bank financing in the U.S. or financing from other U.S. federally regulated entities, we currently have access to equity financing through the private markets in Canada and in the United States. Since the use of marijuana is illegal under U.S. federal law, and in light of concerns in the banking industry regarding money laundering and other federal financial crime related to marijuana, U.S. banks have been reluctant to accept deposit funds from businesses involved with the marijuana industry. Consequently, businesses involved in the marijuana industry often have difficulty finding a bank willing to accept their business. Likewise, marijuana businesses have limited, if any, access to credit card processing services. As a result, marijuana businesses in the U.S. are largely cash-based. This complicates the implementation of financial controls and increases security issues.
Commercial banks, private equity firms and venture capital firms have approached the cannabis industry cautiously to date. However, there are increasing numbers of high net worth individuals and family offices that have made meaningful investments in companies and projects similar to our projects. Although there has been an increase in the amount of private financing available over the last several years, there is neither a broad nor deep pool of institutional capital that is available to cannabis license holders and license applicants. There can be no assurance that additional financing, if raised privately, will be available to us when needed or on terms which are acceptable. Our inability to raise financing to fund capital expenditures or acquisitions could limit our growth and may have a material adverse effect upon future profitability. See “Risk Factors”.
State-Level Overview
The following sections present an overview of market and regulatory conditions for the marijuana industry in the state of Nevada, in which we have an operating presence in, and is presented as of July 2019, unless otherwise indicated. Although our activities are compliant with applicable United States state and local law, strict compliance with state and local laws with respect to cannabis may neither absolve the Company of liability under United States federal law, nor may it provide a defense to any federal proceeding which may be brought against the Company.
Nevada Summary
Nevada has a medical marijuana program and passed an adult-use legalization through the ballot box in November 2016. In 2000, Nevada voters passed an amendment to the Nevada state constitution allowing physicians to recommend cannabis for an inclusive set of qualifying conditions including chronic pain and created a limited non-commercial medical marijuana patient/caregiver system. Senate Bill 374, which passed the legislature and was signed by the Governor in 2013, expanded this program and established a for-profit regulated medical marijuana industry.
The Nevada Division of Public and Behavioral Health licensed medical marijuana establishments up until July 1, 2017 when the state’s medical marijuana program merged with adult-use marijuana enforcement under the NV DOT. In 2014, Nevada accepted medical marijuana business applications and a few months later the Division approved 182 cultivation licenses, 118 licenses for the production of edibles and infused products, 17 independent testing laboratories, and 55 medical marijuana dispensary licenses. The number of dispensary licenses was then increased to 66 by legislative action in 2015. From September 7, 2018 to September 20,2018 Nevada began accepting retail marijuana business applications and shortly thereafter in December, the State of Nevada awarded sixty-one (61) retail marijuana store licenses. The application process is merit-based, competitive, and is currently closed. Residency is not required to own or invest in a Nevada medical cannabis business. In addition, vertical integration is neither required nor prohibited. Nevada’s medical law includes patient reciprocity, which permits medical patients from other states to purchase marijuana from Nevada dispensaries. Nevada also allows for dispensaries to deliver medical marijuana to patients.
Each medical marijuana establishment must register with the NV DOT and apply for a medical marijuana establishment registration certificate. Among other requirements, there are minimum liquidity requirements and restrictions on the geographic location of a medical marijuana establishment as well as restrictions relating to the age and criminal background of employees, owners, officers and board members of the establishment. All employees must be over 21 and all owners, officers and board members must not have any previous felony convictions or had a previously granted medical marijuana registration revoked. Additionally, each volunteer, employee, owner, officer and board member of a medical marijuana establishment must be registered with the NV DOT as a medical marijuana agent and hold a valid medical marijuana establishment agent card. The establishment must have adequate security measures and use an electronic verification system and inventory control system. If the proposed medical marijuana establishment will sell or deliver edible marijuana products or marijuana-infused products, proposed operating procedures for handling such products which must be preapproved by the NV DOT.
In response to the rescission of the Cole Memorandum, former Nevada Attorney General Adam Laxalt has issued a public statement, pledging to defend the law after it was approved by voters. Former Nevada Governor Brian Sandoval also stated, “Since Nevada voters approved the legalization of recreational marijuana in 2016, I have called for a well-regulated, restricted and respected industry. My administration has worked to ensure these priorities are met while implementing the will of the voters and remaining within the guidelines of both the Cole and Wilkinson federal memos,” and that he would like for Nevada to follow in the footsteps of Colorado, where the U.S. attorneys do not plan to change the approach to prosecuting crimes involving recreational marijuana.
To our knowledge, there have not been any additional statements or guidance made by federal authorities or prosecutors regarding the risk of enforcement action in Nevada.
In determining whether to issue a medical marijuana establishment registration certificate pursuant to NRS 453A.322, the NV DOT, in addition the application requirements set out, considers the following criteria of merit:
|
|
(a) |
the total financial resources of the applicant, both liquid and illiquid; |
|
|
(b) |
the previous experience of the persons who are proposed to be owners, officers or board members of the proposed medical marijuana establishment at operating other businesses or non- profit organizations; |
|
|
(c) |
the educational achievements of the persons who are proposed to be owners, officers or board members of the proposed medical marijuana establishment; |
|
|
(d) |
any demonstrated knowledge or expertise on the part of the persons who are proposed to be owners, officers or board members of the proposed medical marijuana establishment with respect to the compassionate use of marijuana to treat medical conditions; |
|
|
(e) |
whether the proposed location of the proposed medical marijuana establishment would be convenient to serve the needs of persons who are authorized to engage in the medical use of marijuana; |
|
|
(f) |
the likely impact of the proposed medical marijuana establishment on the community in which it is proposed to be located; |
|
|
(g) |
the adequacy of the size of the proposed medical marijuana establishment to serve the needs of persons who are authorized to engage in the medical use of marijuana; |
|
|
(h) |
whether the applicant has an integrated plan for the care, quality and safekeeping of medical marijuana from seed to sale; |
|
|
(i) |
the amount of taxes paid to, or other beneficial financial contributions made to, the State of Nevada or its political subdivisions by the applicant or the persons who are proposed to be owners, officers or board members of the proposed medical marijuana establishment; and |
|
|
(j) |
any other criteria of merit that the Division determines to be relevant. |
A medical marijuana establishment registration certificate expires one year after the date of issuance and may be renewed upon resubmission of the application information and renewal fee to the NV DOT.
Governor Sisolak has signed multiple Assembly Bills and Senate Bills having to do with or affecting both retail and medical aspects in the marijuana industry. Specifically, Senate Bill 430 effects the medical marijuana industry, amending NRS 453A.050 to further expand the definition of chronic or debilitating medical condition as it is defined in relation to the medical use of marijuana. The new definition includes: an anxiety disorder, autism spectrum disorder, autoimmune disease, dependence upon opioids, anorexia, medical condition related to acquired immune deficiency syndrome (AIDS) or the human immunodeficiency virus (HIV) and a neuropathic condition. NRS 453A.050 continues to protect a person who holds a valid registry identification card or letter of approval from state prosecution for possession, delivery and production of marijuana.
Adult-Use Retail Marijuana Program
The sale of marijuana for adult-use in Nevada was approved by ballot initiative on November 8, 2016. Nevada Revised Statute 453D exempts a person who is 21 years of age or older from state or local prosecution for possession, use, consumption, purchase, transportation or cultivation of certain amounts of marijuana and requires the NV DOT to begin receiving applications for the licensing of marijuana establishments on or before January 1, 2018.
In February 2017, the Nevada Department of Taxation announced plans to issue “early start” recreational marijuana establishment licenses in the summer of 2017. Beginning on July 1, 2017, these licenses allowed marijuana establishments holding both a retail marijuana store and dispensary license to sell their existing medical marijuana inventory as either medical or adult-use marijuana, and expired at the end of the year. Starting July 1, 2017, medical and adult-use marijuana have incurred a 15% excise tax on the first wholesale sale (calculated on the fair market value) and adult-use cannabis have incurred an additional 10% special retail marijuana sales tax in addition to any general state and local sales and use taxes. Effective July 1, 2019, revenue collected from the 10% excise tax on retail marijuana stores will be deposited into the State Distributive School Account in the State General Fund.
On July 1, 2020, portions of Assembly Bill 533 will go into effect. Amongst the provisions of AB 533 that will go into effect are any person who owns more than five percent (5%) ownership interest in a marijuana establishment has to obtain a cannabis establishment agent registration card for a cannabis executive.
On January 16, 2018, the Marijuana Enforcement Division of the NV DOT issued final rules governing its adult-use marijuana program, pursuant to which up to sixty-six (66) permanent adult-use marijuana dispensary licenses will be issued. Existing adult-use marijuana licensees under the “early start” regulations must re-apply for licensure under the permanent rules in order to continue adult-use sales.
Under Nevada’s adult-use marijuana law, the NV DOT licenses marijuana cultivation facilities, product manufacturing facilities, distributors, retail stores and testing facilities. After merging medical and adult-use marijuana regulation and enforcement, the single regulatory agency is now known as the “Marijuana Enforcement Division of the Department of Taxation.” For the first 18 months, applications to the Department for adult-use establishment licenses can only be accepted from existing medical marijuana establishment certificate holders and existing liquor distributors for the adult-use distribution license.
There are five types of retail marijuana establishment licenses under Nevada's retail marijuana program:
|
|
1. |
Cultivation Facility - licensed to cultivate (grow), process, and package marijuana; to have marijuana tested by a testing facility; and to sell marijuana to retail marijuana stores, to marijuana product manufacturing facilities, and to other cultivation facilities, but not to consumers. |
|
|
2. |
Distributor - licensed to transport marijuana from a marijuana establishment to another marijuana establishment. For example, from a cultivation facility to a retail store. |
|
|
3. |
Product Manufacturing Facility - licensed to purchase marijuana; manufacture, process, and package marijuana and marijuana products; and sell marijuana and marijuana products to other product manufacturing facilities and to retail marijuana stores, but not to consumers. Marijuana products include things like edibles, ointments, and tinctures. |
|
|
4. |
Testing Facility - licensed to test marijuana and marijuana products, including for potency and contaminants. |
|
|
5. |
Retail Store - licensed to purchase marijuana from cultivation facilities, marijuana and marijuana products from product manufacturing facilities, and marijuana from other retail stores; can sell marijuana and marijuana products to consumers. |
Administration of the regular retail program in Nevada is governed by permanent regulations. The NV DOT conducted public consultation and received public comments on the Revised Proposed Adult-Use Marijuana Regulation (LCB File No. R092-17) dated December 13, 2017 (the “Nevada Adult-Use Regulation”). On February 27, 2018, the NV DOT adopted the Nevada Adult- Use Regulations and the NV DOT began accepting applications for adult-use marijuana registration certificates shortly thereafter. In December of 2018, the Department of Taxation awarded 61 retail marijuana store licenses throughout the State of Nevada.
In determining who shall receive a license for a retail marijuana store in response to the request for applications made pursuant to NAC 453D.260, the Department will rank the applications… in order from first to last based on compliance with NAC 453D and chapter 453D of NRS and on the following content:
|
|
a. |
Whether the owners, officers or board members have experience operating another kind of business that has given them experience which is applicable to the operation of a marijuana establishment; |
|
|
b. |
The diversity of the owners, officers or board members of the proposed marijuana establishment; |
|
|
c. |
The educational achievements of the owners, officers or board members of the proposed marijuana establishment; |
|
|
d. |
The financial plan and resources of the applicant, both liquid and illiquid; |
|
|
e. |
Whether the applicant has an adequate integrated plan for the care, quality and safekeeping of marijuana from seed to sale; |
|
|
f. |
The amount of taxes paid and other beneficial financial contributions, including, without limitation, civic or philanthropic involvement with this State or its political subdivisions, by the applicant or the owners, officers or board members of the proposed marijuana establishment; |
|
|
g. |
Whether the owners, officers or board members of the proposed marijuana establishment have direct experience with the operation of a medical marijuana establishment or marijuana establishment in this State and demonstrated a record of operating such an establishment in compliance with the laws and regulations of this State for an adequate period of time to demonstrate success; |
|
|
h. |
The experience of key personnel that the applicant intends to employ in operating the type of marijuana establishment for which the applicant seeks a license; and |
|
|
i. |
Any other criteria that the Department determines to be relevant. |
In response to the ever-changing marijuana industry, Governor Sisolak has passed Assembly Bills: 132, 466, and 533 along with Senate Bills: 346, and 545, amongst others, all relating to the marijuana industry in the State of Nevada.
Assembly Bill 132, which will go in to effect on January 1, 2020, provides that it is unlawful for an employer to refuse/fail to hire a prospective employee who submitted to a drug test and the results showed a presence of marijuana. AB 132 does not apply to persons applying to be a firefighter or medical tech, whom operates a motor vehicle or a person whose employment affects the safety of others.
Assembly Bill 466 requires the creation of a pilot program to facilitate certain financial transactions relating to marijuana. AB 466 is authorized to begin October 1, 2019 and is set to expire, by limitation, on June 23, 2023. The goals of AB 466 are to give marijuana establishments a financial institution that will allow them to continue to strive towards reducing the risk to the safety and welfare of the public that is seen when large sums of cash are present, provide marijuana establishments with a safe way to pay taxes, prevent revenue from going to criminal enterprises and prevent the distribution of marijuana to minors. AB 466 has built in reporting provisions which state that the State Treasurer shall submit to the Director of the Legislative Counsel Bureau a report about the pilot program before December 1, 2020 and every six (6) months thereafter.
Assembly Bill 533 was approved by Governor Sisolak on June 12, 2018. Included in AB 533 is Section 52 which calls for the creation of the Cannabis Advisory Commission (CAM) and the Cannabis Compliance Board (CCB). The CAM shall be comprised of Officers and Members appointed by the Governor. The purpose of the CAM is to study issues and make recommendations to the CCB in regard to cannabis regulations. Additionally, the CAM will recommend to the CAB any guidelines, rules or regulations or changes to existing ones. Furthermore, the CAM will study the distribution of licenses, emerging technologies for collecting data and recommend to the board any statutory changes that the Commission determines to be appropriate. The Cannabis Compliance Board (CCB) is created as a part of Section 54 of Assembly Bill 533. AB 533 calls for the authority to license and regulate persons and establishments involved in the marijuana industry in this State to be transferred to the Cannabis Compliance Board. The CCB will consist of five (5) members who will be appointed by Governor Sisolak. Governor Sisolak has modeled the CCB after the successful Nevada Gaming Control Board. The CCB will license, register and regulate marijuana establishments and those who are engaged in the production and/or sale of cannabis and cannabis products. Additionally, section 65 of AB 533 outlines the procedures by which the CCB can adopt regulations and provides the procedure by which the Legislative Commission can review those regulations. Section 57 of AB 533 outlines that the CCB can perform certain audits of the accounts, programs, funds, activities, and functions of the licensees or they are authorized to require the Department of Taxation to do so. Section 68 provides the procedures for disciplinary actions if a marijuana establishment violates any provision or has an unsatisfactory audit.
Section 178 of Assembly Bill 533 which will go into effect on July 1, 2020 further expands on the concept that a person who is 21 years of age or older is exempt from state prosecution for:
|
|
A. |
The possession, delivery or production of cannabis; |
|
|
B. |
The possession or delivery of paraphernalia; |
|
|
C. |
Aiding and abetting another in the possession, delivery or production of cannabis; |
|
|
D. |
Aiding and abetting another in the possession or delivery of paraphernalia; |
|
|
E. |
Any combination of the acts described in paragraphs (a) to (d), inclusive; and |
|
|
F. |
Any other criminal offense in which the possession, delivery or production of cannabis or the possession or delivery of paraphernalia is an element. |
The legislative intent behind Section 178 is to provide protections for persons and establishments engaged in certain actions relating to the adult use of cannabis. Section 178 extends the provision of no state prosecution to persons being in the presence or vicinity of the adult use of cannabis in accordance with the provisions of this title.
In addition to the Assembly Bills passed, Governor Sisolak also passed various Senate Bills that are to do with the marijuana industry. As mentioned below in Training, Senate Bill (SB) 346 allows for an independent contractor to enter into a contract to provide training of medical marijuana establishment and marijuana establishment agents.
Senate Bill 430 amends NRS 453A.050 to further expand the definition of chronic or debilitating medical condition as it is defined in relation to the medical use of marijuana. The new definition includes: an anxiety disorder, autism spectrum disorder, autoimmune disease, dependence upon opioids, anorexia, medical condition related to acquired immune deficiency syndrome (AIDS) or the human immunodeficiency virus (HIV) and a neuropathic condition. As mentioned previously, NRS 453A.050 continues to protect a person lawfully consuming medical marijuana from state prosecution for the possession, delivery or production of marijuana.
License and Regulations
In the state of Nevada, only cannabis that is grown or produced in the state by a licensed establishment may be sold in the state. The Nevada regulatory regime is not a vertically integrated system and only permits the holder of a retail dispensary license and registration certificate to purchase marijuana from cultivation facilities, marijuana and marijuana products from product manufacturing facilities and marijuana from other retail stores, and allows the sale of marijuana and marijuana products to consumers.
A medical cultivation license permits its holder to acquire, possess, cultivate, deliver, transfer, have tested, transport, supply or sell marijuana and related supplies to medical marijuana dispensaries, facilities for the production of edible medical marijuana products and/or medical marijuana-infused products, or other medical marijuana cultivation facilities.
A retail cultivation license permits its holders to acquire, possess, cultivate, deliver, transfer, have tested, transport, supply or sell marijuana and related supplies to retail marijuana stores, retail marijuana production facilities for the production of edible, marijuana products and/or marijuana infused products or other retail marijuana cultivation facilities.
The medical product manufacturing license permits its holder to acquire, possess, manufacture, deliver, transfer, transport, supply, or sell edible marijuana products or marijuana infused products to other medical marijuana production facilities or medical marijuana dispensaries.
The retail product manufacturing license permits its holder to acquire, possess, manufacture, deliver, transfer, transport, supply, or sell edible marijuana products or marijuana infused products to other retail marijuana production facilities or retail marijuana stores.
Reporting Requirements
The state of Nevada uses a computerized track and trace system used to track commercial cannabis activity and seed-to-sale. Individual licensees, whether directly or through third-party integration systems, are required to push data to the state to meet all reporting requirements. See section entitled “Compliance with Applicable State Law in the United States” below.)
Storage and Security
To ensure the safety and security of cannabis business premises and to maintain adequate controls against the diversion, theft, and loss of cannabis or cannabis products, Nevada state law requires the following:
|
|
(a) |
be an enclosed, locked facility; |
|
|
(b) |
have a single secure entrance; |
|
|
(c) |
train employees in security measures and controls, emergency response protocol, confidentiality requirements, safe handling of equipment, procedures for handling products, as well as the differences in strains, methods of consumption, methods of cultivation, methods of fertilization and methods for health monitoring; |
|
|
(d) |
install security equipment to deter and prevent unauthorized entrances, which includes: |
|
|
a. |
devices that detect unauthorized intrusion which may include a signal system; and |
|
|
b. |
exterior lighting to facilitate surveillance; |
|
|
(e) |
electronic monitoring must be in place, which includes: |
|
|
a. |
at least one call-up monitor that is 19 inches or more; |
|
|
b. |
a video printer capable of immediately producing a clear still photo from any video camera image;
|
|
|
c. |
video cameras with recording resolution of at least 1920 x 1080, or the equivalent, at a rate of at least 15 frames per second, which records 24 hours a day and is capable of being accessed remotely by a law enforcement agency in real time upon request. |
|
|
d. |
video cameras with a recording resolution of at least 720 x 480 which provides coverage of all entrances to and exits from limited access areas and all entrances to and exits from the building and which can identify any activity occurring in or adjacent to the building; |
|
|
e. |
a video camera at each point-of-sale location which allows for the identification of any person who holds a valid registry identification card, including, without limitation, a designated primary caregiver, purchasing medical marijuana; |
|
|
f. |
a video camera in each grow room which can identify any activity occurring within the grow room in low light conditions; |
|
|
g. |
a method for storing video recordings from the video cameras for at least thirty (30) calendar days; |
|
|
h. |
a failure notification system that provides an audible and visual notification of any failure in the electronic monitoring system; |
|
|
i. |
sufficient battery backup for video cameras and recording equipment to support at least five (5) minutes of recording in the event of a power outage; and |
|
|
j. |
security alarm to alert local law enforcement of unauthorized breach of security; and |
|
|
(f) |
implement security procedures that: |
|
|
a. |
restrict access of the establishment to only those persons/employees authorized to be there; |
|
|
b. |
deter and prevent theft; |
|
|
c. |
provide identification (badge) for those persons/employees authorized to be in the establishment; |
|
|
d. |
prevent loitering; |
|
|
e. |
require and explain electronic monitoring; and |
|
|
f. |
require and explain the use of automatic or electronic notification to alert local law enforcement of an unauthorized breach of security. |
Training
As of January 2, 2020, in accordance with SB 346, an independent contractor will be authorized to enter into a contract to provide training of medical marijuana establishment agents and marijuana establishment agents. The independent contractor is required to submit a plan to the Department of Taxation describing the manner their training will be conducted.
Transportation
In Nevada, marijuana may only be transported from a licensed grow or production facility by a licensed marijuana distributor. Prior to transporting the marijuana or marijuana products, the distributor must complete a trip plan which includes: the agent name and registration number providing and receiving the marijuana; the date and start time of the trip; a description, including the amount, of the marijuana or marijuana products being transported; and the anticipated route of transportation.
During the transportation of marijuana or marijuana products, the licensed marijuana distributor agent must: (a) carry a copy of the trip plan with him or her for the duration of the trip; (b) have his or her marijuana establishment agent card in his or her immediate possession; (c) use a vehicle without any identification relating to marijuana and which is equipped with a secure lockbox or locking cargo area which must be used for the sanitary and secure transportation of marijuana, or marijuana products; (d) have a means of communicating with the marijuana establishment for which he or she is providing the transportation; and (e) ensure that all marijuana or marijuana products are not visible. After transporting marijuana or marijuana products, a licensed marijuana distributor agent must enter the end time of the trip and any changes to the trip plan that was completed.
Each licensed marijuana distributor agent transporting marijuana or marijuana products must report any: (a) vehicle accident that occurs during the transportation to a person designated by the marijuana distributor to receive such reports within two (2) hours after the accident occurs; and (b) loss or theft of marijuana or marijuana products that occurs during the transportation to a person designated by the marijuana distributor to receive such reports immediately after the marijuana establishment agent becomes aware of the loss or theft. A marijuana distributor that receives a report of loss or theft pursuant to this paragraph must immediately report the loss or theft to the appropriate law enforcement agency and to the NV DOT. The distributor must report any unauthorized stop that lasts longer than two (2) hours to the NV DOT.
A marijuana distributor shall maintain the required documents and provide a copy of the documents required to the NV DOT for review upon request. Each marijuana distributor shall maintain a log of all received reports.
Employees of licensed marijuana distributors, including drivers transporting marijuana and marijuana products, must be 21 years of age or older and must obtain a valid marijuana establishment agent registration card issued by the NV DOT. If a marijuana distributor is co-located with another type of business, all employees of co-located businesses must have marijuana establishment agent registration cards unless the co-located business does not include common entrances, exits, break room, restrooms, locker rooms, loading docks, and other areas as are expedient for business and appropriate for the site as determined and approved by Department inspectors. While engaged in the transportation of marijuana and marijuana products, any person that occupies a transport vehicle when it is loaded with marijuana or marijuana products must have their physical marijuana establishment agent registration card in their possession.
All drivers must carry in the vehicle valid driver’s insurance at the limits required by the State of Nevada and the NV DOT. All drivers must be bonded in an amount sufficient to cover any claim that could be brought, or disclose to all parties that their drivers are not bonded. Marijuana establishment agent registration cardholders and the licensed marijuana distributor they work for are responsible for the marijuana and marijuana product once they take control of the product and leave the premises of the marijuana establishment.
There is no load limit on the amount or weight of marijuana and marijuana products that are being transported by a licensed marijuana distributor. Marijuana distributors are required to adhere to NV DOT regulations and those required through their insurance coverage. When transporting by vehicle, marijuana and marijuana product must be in a lockbox or locked cargo area. A trunk of a vehicle is not considered secure storage unless there is no access from within the vehicle and it is not the same key access as the vehicle. Live plants can be transported in a fully enclosed, windowless locked trailer or secured area inside the body/compartment of a locked van or truck so that they are not visible to the outside. If the value of the marijuana and marijuana products being transported by vehicle is in excess of $10,000 (the insured value per the shipping manifest), the transporting vehicle must be equipped with a car alarm with sound or have no less than two (2) of the marijuana distributor’s marijuana establishment agent registration cardholders involved in the transportation. All marijuana and marijuana product must be tagged for purposes of inventory tracking with a unique identifying label as required by the NV DOT and remain tagged during transport. This unique identifying label should be similar to the stamp for cigarette distribution. All marijuana and marijuana products when transported by vehicle must be transported in sealed packages and containers and remain unopened during transport. All marijuana and marijuana product transported by vehicle should be inventoried and accounted for in the inventory tracking system. Loading and unloading of marijuana and marijuana products from the transporting vehicle must be within view of existing video surveillance systems prior to leaving the origination location. Security requirements are required for the transportation of marijuana and marijuana products.
Oasis LLC Licenses
Oasis is licensed to operate in the City of Las Vegas as a Dual Use Marijuana Business and in the State of Nevada as a Medical Marijuana Dispensary Establishment and a Retail Marijuana Store. City Trees Production is licensed to operate in the state of Nevada as a Medical Marijuana Production Establishment, a Retail Marijuana Product Manufacturing facility and a Retail Marijuana Distributor. City Trees Production is licensed to operate in the state of Nevada as a Medical Marijuana Cultivation Facility and a Retail Marijuana Cultivator. The table below lists the licenses issued to the Oasis LLCs in respect of the Oasis LLCs’ operations in Nevada (including municipal licenses). Under applicable laws, the licenses permit the Oasis LLCs to cultivate, manufacture, process, package, sell, and purchase marijuana pursuant to the terms of the licenses, which are issued by the NV DOT under the provisions of Nevada Revised Statutes section 453A, 453D, their associated sections of the Nevada Administrative Code and local regulations pertaining to cannabis businesses. All provisional licenses owned by Oasis are, as of the date hereof, active with the state of Nevada. All licenses are independently issued for each approved activity for use at the Oasis LLCs’ facilities in Nevada.
All marijuana establishments must register with the NV DOT. If applications contain all required information and after vetting by officers, establishments are issued a medical marijuana establishment registration certificate. In a local governmental jurisdiction that issues business licenses, the issuance by the NV DOT of a medical marijuana establishment registration certificate is considered provisional until the local government has issued a business license for operation and the establishment is in compliance with all applicable local governmental ordinances. Final registration certificates are valid for a period of one year and are subject to annual renewals after required fees are paid and the business remains in good standing. Renewal requests are typically communicated through email from NV DOT and include a renewal form. The renewal periods serve as an update for NV DOT on the licensee’s status toward active licensure. Maintaining the licenses in good standing is critical to the success of a marijuana business in Nevada. Failure to adhere to the regulations can result in significant fines and penalties, including the suspension or revocation of the license.
The licenses are independently issued for each approved activity for use at Oasis LLC facilities. The table below lists the licenses issued to the Oasis LLCs in respect of their operations in Nevada.
Licenses in the State of Nevada
|
Holding Entity |
Permit/License |
Location City |
Expiration/Renewal Date |
Description |
|
Serenity Wellness Center LLC d/b/a Oasis Cannabis |
D90 - Medical Marijuana Dispensary License #: M66-00051 |
Las Vegas |
08/27/2019 |
City of Las Vegas Marijuana Business License for a Medical Dispensary |
|
Serenity Wellness Center LLC d/b/a Oasis Cannabis |
R90 - Retail Marijuana Store (Rec Sales) License # P65-00138 |
Las Vegas |
07/01/2019* *Renews every 90 days |
City of Las Vegas Marijuana Business License for a Retail Marijuana Store |
|
Serenity Wellness Center LLC d/b/a Oasis Medical Cannabis |
Medical Marijuana Registration Certificate: # 02916424476864783141 MME Code: D046 |
|
06/30/2020 |
State of NV Final Registration Certificate – Medical Marijuana Dispensary Establishment |
|
Serenity Wellness Center LLC d/b/a Oasis Medical Cannabis |
Retail Marijuana Store License #: 55910347793434478299 ME Code: RD046 |
|
06/30/2020 |
State of NV – Retail Marijuana Store License |
|
Oasis Cannabis |
G50 – General Retail Sales Drug Paraphernalia License #: G66-07378 |
Las Vegas |
08/01/2019 |
City of Las Vegas general retail sales license |
|
Oasis Cannabis |
G50 – General Retail Sales Drug Paraphernalia License #: G64-01243 |
Las Vegas |
08/01/2019 |
City of Las Vegas general retail sales license |
|
Community Oasis LLC |
A51 – Automated Teller Operator License #: G63-09197 |
Las Vegas |
12/01/2019 |
City of Las Vegas license to operate an automated teller |
|
Community Oasis LLC |
I50 – Instruction Services Workshops/Yoga/Art Sales |
Las Vegas |
09/01/2019 |
City of Las Vegas license to operate an instructional services business |
|
Serenity Wellness Products LLC d/b/a City Trees |
MM08 Production – GS License #: 105437 |
North Las Vegas |
07/31/2019* *Renews every 90 days |
City of North Las Vegas Marijuana Production License |
|
Serenity Wellness Products LLC d/b/a City Trees |
TME08 Rec Production – GS License #: 111296 |
North Las Vegas |
07/31/2019 *Renews every 90 days |
City of North Las Vegas Marijuana Production License |
|
Serenity Wellness Products LLC d/b/a City Trees |
TME 11 Marijuana Distributor License #: 113029 |
North Las Vegas |
01/31/2020 |
City of North Las Vegas license to distribute marijuana in the City. |
|
Serenity Wellness Products LLC d/b/a City Trees |
Medical Marijuana Registration Certificate: # 40297970315350477547 MME Code: P024 |
|
06/30/2020 |
State of NV Final Registration Certificate – Medical Marijuana Production Establishment |
|
Serenity Wellness Products LLC d/b/a City Trees |
Retail Marijuana Product Manufacturing License #: 79484750509886968559 ME Code: RP024 |
|
06/30/2020 |
State of NV Retail Marijuana Product Manufacturing License |
|
Serenity Wellness Products LLC d/b/a City Trees |
Retail Marijuana Distributor License #: 61611537222691531848 ME Code: T073 |
|
06/30/2020 |
State of NV Retail Marijuana Distributor License |
|
Serenity Wellness Products LLC d/b/a City Trees |
Medical Marijuana Production Facility OLV Marijuana Production License #: M65-00015 |
|
07/01/2019 *Renews every 90 days |
City of Las Vegas license required to sell to dispensaries within its jurisdiction |
|
Serenity Wellness Growers LLC d/b/a City Trees |
MM02 Cultivation - GS License #: 105436 |
North Las Vegas |
07/31/2019* *Renews every 90 days |
City of North Las Vegas Marijuana Cultivation License |
|
Serenity Wellness Growers LLC d/b/a City Trees |
TME02 Rec Cultivation – GS License #: 111295 |
North Las Vegas |
07/31/2019 *Renews every 90 days |
City of North Las Vegas Marijuana Cultivation License |
|
Serenity Wellness Growers LLC d/b/a City Trees |
Medical Marijuana Registration Certificate: 36161311931874315998 MME Code: C039 |
|
06/30/2020 |
State of NV Medical Marijuana Cultivation Facility Registration Certificate |
|
Serenity Wellness Growers LLC d/b/a City Trees |
Retail Marijuana Cultivator License #: 77486514896179438118 ME Code: RC039 |
|
06/30/2020 |
State of NV Retail Marijuana Cultivator License |
|
Serenity Wellness Growers LLC d/b/a City Trees |
X90 – Medical Marijuana Cultivation Facility OLV License #: M65-00014 |
Las Vegas |
07/01/2019 *Renews every 90 days |
City of Las Vegas license required to sell marijuana within its jurisdiction |
Nevada License and Regulations
The retail dispensary licenses permit the Oasis LLCs to purchase marijuana from Nevada licensed cultivation facilities, marijuana and marijuana products from Nevada licensed product manufacturing facilities and marijuana from other Nevada licensed retail stores and allows the sale of marijuana and marijuana products to consumers. No marijuana or marijuana infused products may be brought into Nevada from outside of Nevada. Unlicensed marijuana activities are subject to harsh criminal penalties under Nevada state law.
The medical and retail cultivation licenses permit the Oasis LLCs to acquire, possess, cultivate, deliver, transfer, have tested, transport, supply or sell marijuana and related supplies to Nevada licensed medical and retail marijuana dispensaries, facilities for the production of edible medical marijuana products and/or medical marijuana-infused products, or other medical and retail marijuana cultivation facilities.
The medical and retail product manufacturing license permits the Oasis LLCs to acquire, possess, manufacture, deliver, transfer, transport, supply, or sell edible marijuana products or marijuana infused products to other Nevada licensed medical and retail marijuana production facilities or medical and retail marijuana dispensaries.
As related to the recent retail marijuana store license application process completed by the State of Nevada in December, 2018, there are currently lawsuits surrounding the awarding of licenses in conjunction with the application period.1
Nevada Reporting Requirements
The state of Nevada uses METRC as the state’s computerized T&T system for seed-to-sale. Individual licensees whether directly or through third-party integration systems are required to push data to the state to meet all reporting requirements. The Oasis LLCs have designated an in-house computerized seed to sale software that integrate with METRC via API (GreenBits), which captures the required data points for cultivation, manufacturing and retail as required in Nevada Revised Statutes section 453A.
Compliance with Applicable State Law in the United States
We, via the Oasis LLCs, are classified as having a “direct” involvement in the U.S. marijuana industry and are in compliance with applicable licensing requirements and the regulatory framework enacted by the state of Nevada. Neither the Company nor the Oasis LLCs are subject to any citations or notices of violation with applicable licensing requirements and the regulatory framework enacted by each applicable U.S. state which may have an impact on its licenses, business activities or operations.
We have in place a detailed compliance program overseen and maintained by external state and local regulatory/compliance counsel. Our internal compliance team (consisting of managers for each respective business unit) implements the compliance program.
Our internal compliance team oversees training for all employees, including on the following topics:
|
|
● |
compliance with state and local laws |
|
|
● |
safe cannabis use |
|
|
● |
dispensing procedures |
|
|
● |
security and safety policies and procedures |
|
|
● |
inventory control |
|
|
● |
quality control |
|
|
● |
transportation procedures |
Our compliance program emphasizes security and inventory control to ensure strict monitoring of cannabis and inventory from delivery by a licensed distributor to sale or disposal. Only authorized, properly trained employees are allowed to access the Company’s computerized seed-to-sale system.
Our internal compliance team, together with external state and local regulatory/compliance counsel, monitors all compliance notifications from the regulators and inspectors in each market, timely resolving any issues identified. We keep records of all compliance notifications received from the state regulators or inspectors and how and when the issue was resolved.
Further, we have created comprehensive standard operating procedures that include detailed descriptions and instructions for receiving shipments of inventory, inventory tracking, recordkeeping and record retention practices related to inventory, as well as procedures for performing inventory reconciliation and ensuring the accuracy of inventory tracking and recordkeeping. We maintain accurate records of our inventory at all licensed facilities. Adherence to our standard operating procedures is mandatory and ensures that our operations are compliant with the rules set forth by the applicable state and local laws, regulations, ordinances, licenses and other requirements. We ensure adherence to standard operating procedures by regularly conducting internal inspections and ensure that any issues identified are resolved quickly and thoroughly.
1A CLS Holdings USA, Inc., subsidiary, Serenity Wellness Center LLC is currently involved in the litigation concerning the Department of Taxation issuance of conditional retail marijuana store licenses. Case No.: A-19-786962-B. (Serenity Wellness Center, LLC, et al., Plaintiffs, vs. State of Nevada, Department of Taxation, Defendant, and Nevada Organic Remedies, LLC, a Nevada limited liability company; Greenmart of Nevada NLV LLC, a Nevada limited liability company; Integral Associates LLC d/b/a Essence Cannabis Dispensaries, a Nevada limited liability company; Essence Tropicana, LLC, a Nevada limited liability company; Essence Henderson, LLC, a Nevada limited liability company; CPCM Holdings, LLC d/b/a Thrive Cannabis Marketplace, Commerce Park Medical, LLC, a Nevada limited liability company; and Cheyenne Medical, LLC, a Nevada limited liability company, Defendants – Intervenors).
In January 2018, former United States Attorney General, Jeff Sessions rescinded the Cole Memorandum and thereby created a vacuum of guidance for enforcement agencies and the Department of Justice.2 As an industry best practice, despite the recent rescission of the Cole Memorandum, the Company continues to do the following to ensure compliance with the guidance provided by the Cole Memorandum:
|
|
● |
Ensure the operations of its subsidiaries are compliant with all licensing requirements that are set forth with regards to cannabis operation by the applicable state, county, municipality, town, township, borough, and other political/administrative divisions. To this end, the Company retains appropriately experienced legal counsel to conduct the necessary due diligence to ensure compliance of such operations with all applicable regulations; |
|
|
● |
the Company only works through licensed operators, which must pass a range of requirements, adhere to strict business practice standards and be subjected to strict regulatory oversight whereby sufficient checks and balances ensure that no revenue is distributed to criminal enterprises, gangs and cartels; and |
|
|
● |
we conduct reviews of products and product packaging to ensure that the products comply with applicable regulations and contain necessary disclaimers about the contents of the products to prevent adverse public health consequences from cannabis use and prevent impaired driving. |
We, together with external state and local regulatory/compliance counsel, will continue to monitor compliance on an ongoing basis in accordance with our compliance program and standard operating procedures. While our operations are in full compliance with all applicable state laws, regulations and licensing requirements, such activities remain illegal under United States federal law. For the reasons described above and the risks further described in the “Risk Factors” section below, there are significant risks associated with the business of the Company. Readers are strongly encouraged to carefully read all of the risk factors contained in the “Risk Factors” section below.
Although state-licensed businesses engaged in such activities are currently proceeding largely free from federal prosecution and recently-enacted federal spending legislation prohibits the Department of Justice from using federal funds to prevent states from implementing their own marijuana laws, changes in congress or in the executive administration, including presidential elections, could result in changes to current federal enforcement policies regarding cannabis-related activities which are legal under certain state laws. Therefore, by operating the business, we will face the possibility of civil and criminal sanctions.
Additionally, certain states in which we seek to operate may prohibit non-resident companies from conducting business directly in the state. In such states, we will seek to enter into a collaborative arrangement with a local entity holding the necessary licensure, whereby we will agree to lease our facilities, equipment and employees to the licensed entity in exchange for a fee. Such an arrangement may be difficult to secure and/or expensive to maintain, as we will be reliant on the licensee to maintain its license in order to continue operations. Further, various state and local licensure application and approval processes may require significant time and expense, and, upon becoming authorized to do business in a state, it may be difficult or expensive for us to comply with the oft-changing laws, regulations and licensure requirements of each state and municipality where we are doing business.
We will need to obtain applicable state licenses in each state in which we will operate processing facilities. License reqirements and procedures vary from state to state. The initial state in which we operate is Nevada. We plan to operate in Massachusetts if and when we exercise the IGH Option.
2 U.S. Dept. of Justice. (2013). Memorandum for all United States Attorneys re: Guidance Regarding Marijuana Enforcement. Washington, DC: US Government Printing Office. Retrieved from https://www.justice.gov/iso/opa/resources/3052013829132756857467.pdf.
PROPERTIES
The mailing address of our principal executive office is 11767 South Dixie Highway, Suite 115, Miami, Florida 33156. We currently maintain an administrative office at 3355 SW 59th Avenue, Miami, Florida 33155. Alternative Solutions and the Oasis LLCs lease space for a dispensary and administrative offices at 1800 Industrial Road, Suites 100, 102, 160 and 180, Las Vegas, Nevada 89102, and for a cultivation and processing facility at 203 E. Mayflower Avenue, North Las Vegas, Nevada 89030.
Our business faces certain risks. The risks described below may not be the only risks we face. Additional risks that we do not yet know of or that we currently think are immaterial may also impair our business. If any of the events or circumstances described as risks below or elsewhere in this report actually occurs, our business, results of operations or financial condition could be materially and adversely affected. In connection with any investment decision, you should carefully consider the following factors, which could materially affect our business, financial condition or results of operations. You should read these Risk Factors in conjunction with “Management’s Discussion and Analysis of Financial Condition and Results of Operations” in Item 7 and our Consolidated Financial Statements and related notes in Item 8.
Risks Related to the Marijuana Industry
Cannabis continues to be a Controlled Substance under the United States Federal Controlled Substances Act and our business may result in federal civil or criminal prosecution.
We are directly engaged in the medical and adult-use cannabis industry in the U.S. where local state law permits such activities however all such activities remain illegal under federal law in the U.S. Investors are cautioned that in the U.S., cannabis is highly regulated at the state level. To our knowledge, there are to date a total of 33 states, and the District of Columbia, Puerto Rico and Guam that have legalized medical cannabis in some form, including California, although not all states have fully implemented their legalization programs. Ten states and the District of Columbia have legalized cannabis for adult use. Fourteen additional states have legalized high-cannabidiol (“CBD”), low Delta-9-tetrahydrocannabinol (“THC”) oils for a limited class of patients. Notwithstanding the permissive regulatory environment of cannabis at the state level, cannabis continues to be categorized as a Schedule I controlled substance under the U.S. Controlled Substance Act of 1970 (codified in 21 U.S.C.A. Section 812) (the “Controlled Substances Act”). Under United States federal law, a Schedule I drug is considered to have a high potential for abuse, no accepted medical use in the United States, and a lack of accepted safety for the use of the substance under medical supervision. Federal law prohibits commercial production and sale of all Schedule I controlled substances, and as such, cannabis-related activities, including without limitation, the importation, cultivation, manufacture, distribution, sale and possession of cannabis remain illegal under U.S. federal law. It is also illegal to aid or abet such activities or to conspire or attempt to engage in such activities. Strict compliance with state and local laws with respect to cannabis may neither absolve us of liability under U.S. federal law, nor provide a defense to any federal proceeding brought against us. An investor’s contribution to and involvement in such activities may result in federal civil and/or criminal prosecution, including, but not limited to, forfeiture of his, her or its entire investment, fines and/or imprisonment.
An appropriations rider contained in the fiscal year 2015, 2016, 2017, 2018 and 2019 Consolidated Appropriations Act provides budgetary constraints on the federal government’s ability to interfere with the implementation of state-based medical cannabis laws. The Ninth Circuit Court of Appeals and other courts have interpreted the language to mean that the U.S. Department of Justice (the “DOJ”) cannot expend funds to prosecute state-law-abiding medical cannabis operators complying strictly with state medical cannabis laws. The Amendment prohibits the federal government from using congressionally appropriated funds to prevent states from implementing their own medical cannabis laws. In February 2019, President Trump renewed the amendment as part of the spending bill and it shall remain valid through September 30, 2019. Continued reauthorization of the Amendment is predicated on future political developments and cannot be guaranteed. If the Amendment expires, federal prosecutors could prosecute even state-compliant medical cannabis operators for conduct within the five-year statute of limitations. The Amendment does not protect state legal adult-use cannabis businesses and the DOJ may spend funds to prosecute persons that are operating in accordance with state adult use cannabis laws.
Violations of any federal laws and regulations could result in significant fines, penalties, administrative sanctions, convictions or settlements arising from civil proceedings conducted by either the federal government or private citizens, or criminal charges and penalties, including, but not limited to, disgorgement of profits, cessation of business activities, divestiture, or prison time. This could have a material adverse effect on us, including our reputation and ability to conduct business, our holding (directly or indirectly) of medical and adult-use cannabis licenses in the U.S., the listing of our securities on the Canadian Securities Exchange (the “CSE”), our financial position, operating results, profitability or liquidity or the market price of our publicly traded shares. In addition, it is difficult for us to estimate the time or resources that would be needed for the investigation or defense of any such matters or our final resolution because, in part, the time and resources that may be needed are dependent on the nature and extent of any information requested by the applicable authorities involved, and such time or resources could be substantial.
The approach to the enforcement of cannabis laws may be subject to change, which creates uncertainty for our business.
As a result of the conflicting views between state legislatures and the federal government regarding cannabis, investments in, and the operations of, cannabis businesses in the U.S. are subject to inconsistent laws and regulations. The so-called “Cole Memorandum” issued by former Deputy Attorney General James Cole on August 29, 2013 and other Obama-era cannabis policy guidance, discussed below, provided the framework for managing the tension between federal and state cannabis laws. Subsequently, as discussed below, former Attorney General Jeff Sessions rescinded the Cole Memo and related policy guidance. Although no longer in effect, these policies, and the enforcement priorities established within, appear to continue to be followed during the Trump administration and remain critical factors that inform the past and future trend of state-based legalization.
The Cole Memo directed U.S. Attorneys not to prioritize the enforcement of federal cannabis laws against individuals and businesses that comply with state medical or adult-use cannabis regulatory programs, provided certain enumerated enforcement priorities (such as diversion or sale of cannabis to minors) were not implicated. In addition to general prosecutorial guidance issued by the DOJ, FinCEN issued a the FinCEN Memorandum on February 14, 2014 outlining Bank Secrecy Act-compliant pathways for financial institutions to service state-sanctioned cannabis businesses, which echoed the enforcement priorities outlined in the Cole Memorandum. On the same day the FinCEN Memorandum was published, the DOJ issued complimentary policy guidance directing prosecutors to apply the enforcement priorities of the Cole Memo when determining whether to prosecute individuals or institutions with crimes related to financial transactions involving the proceeds of cannabis-related activities.
On January 4, 2018, the then Attorney General Jeff Sessions rescinded the Cole Memo, the Cole Banking Memorandum, and all other related Obama-era DOJ cannabis enforcement guidance. While the rescission did not change federal law, as the Cole Memo and other DOJ guidance documents were not themselves laws, the rescission removed the DOJ’s formal policy that state-regulated cannabis businesses in compliance with the Cole Memo guidelines should not be a prosecutorial priority. Notably, former Attorney General Sessions’ rescission of the Cole Memo and the Cole Banking Memorandum has not affected the status of the FinCEN Memorandum issued by the Department of Treasury, which remains in effect. In addition to his rescission of the Cole Memo, former Attorney General Sessions issued a one-page memorandum known as the “Sessions Memorandum.” The Sessions Memorandum explains the DOJ’s rationale for rescinding all past DOJ cannabis enforcement guidance, claiming that Obama-era enforcement policies are “unnecessary” due to existing general enforcement guidance adopted in the 1980s, in chapter 9.27.230 of the U.S. Attorney’s Manual (the “USAM”). The USAM enforcement priorities, like those of the Cole Memo, are based on the use of the federal government’s limited resources and include “law enforcement priorities set by the Attorney General,” the “seriousness” of the alleged crimes, the “deterrent effect of criminal prosecution,” and “the cumulative impact of particular crimes on the community.” Although the Sessions Memorandum emphasizes that cannabis is a federally illegal Schedule I controlled substance, it does not otherwise instruct U.S. Attorneys to consider the prosecution of cannabis-related offenses a DOJ priority, and in practice, most U.S. Attorneys have not changed their prosecutorial approach to date. However, due to the lack of specific direction in the Sessions Memorandum as to the priority federal prosecutors should ascribe to such cannabis activities and the lack of additional guidance since the resignation of former Attorney General Sessions, there can be no assurance that the federal government will not seek to prosecute cases involving cannabis businesses that are otherwise compliant with state law.
Such potential proceedings could involve significant restrictions being imposed upon us or third parties, while diverting the attention of key executives. Such proceedings could have a material adverse effect on our business, revenues, operating results and financial condition as well as our reputation and prospects, even if such proceedings were concluded successfully in our favor. In the extreme case, such proceedings could ultimately involve the criminal prosecution of key executives of the Company, the seizure of corporate assets, and consequently, the inability of the Company to continue its business operations. Strict compliance with state and local laws with respect to cannabis does not absolve the Company of potential liability under U.S. federal law, nor provide a defense to any federal proceeding which may be brought against us. Any such proceedings brought against us may adversely affect our operations and financial performance.
Uncertainty surrounding existing protection from U.S. federal prosecution may adversely affect our operations and financial performance.
Pursuant to the Amendment, until such time as it is not renewed or expires of its own accord, the DOJ is prohibited from expending any funds to prevent states from implementing their own medical cannabis laws. If the Amendment or an equivalent thereof is not successfully included in the next or any subsequent federal omnibus spending bill, the protection which has been afforded thereby to U.S. medical cannabis businesses in the past would lapse, and such businesses would be subject to a higher risk of prosecution under federal law. Although unlikely, there is a possibility that all amendments may be banned from federal omnibus spending bills, and if this occurs and the substantive provisions of the Amendment are not included in the base federal omnibus spending bill or other law, these protections would lapse. To the extent the Amendment is included in a continuing resolution, the protections of the Amendment would lapse if Congress does not reauthorize the resolution or pass another funding measure that includes the Amendment.
We may be in violation of anti-money laundering laws and regulations which could impact our ability to obtain banking services, result in the forfeiture or seizure of our assets and could require us to suspend or cease operations.
We are subject to a variety of laws and regulations domestically and in the U.S. that involve money laundering, financial recordkeeping and proceeds of crime, including the Bank Secrecy Act, as amended by Title III of the Uniting and Strengthening America by Providing Appropriate Tools Required to Intercept and Obstruct Terrorism Act of 2001 (USA PATRIOT Act), the Proceeds of Crime (Money Laundering) and Terrorist Financing Act (Canada), as amended and the rules and regulations thereunder, the Criminal Code (Canada) and any related or similar rules, regulations or guidelines, issued, administered or enforced by governmental authorities in the U.S. and Canada. Since the cultivation, manufacture, distribution and sale of cannabis remains illegal under the Controlled Substances Act, banks and other financial institutions providing services to cannabis-related businesses risk violation of federal anti-money laundering statutes (18 U.S.C. §§ 1956 and 1957), the unlicensed money-remitter statute (18 U.S.C. § 1960) and the Bank Secrecy Act, among other applicable federal statutes. Banks or other financial institutions that provide cannabis businesses with financial services such as a checking account or credit card in violation of the Bank Secrecy Act could be criminally prosecuted for willful violations of money laundering statutes, in addition to being subject to other criminal, civil, and regulatory enforcement actions. Banks often refuse to provide banking services to businesses involved in the cannabis industry due to the present state of the laws and regulations governing financial institutions in the U.S. The lack of banking and financial services presents unique and significant challenges to businesses in the cannabis industry. The potential lack of a secure place in which to deposit and store cash, the inability to pay creditors through the issuance of checks and the inability to secure traditional forms of operational financing, such as lines of credit, are some of the many challenges presented by the unavailability of traditional banking and financial services. These statutes can impose criminal liability for engaging in certain financial and monetary transactions with the proceeds of a “specified unlawful activity” such as distributing controlled substances which are illegal under federal law, including cannabis, and for failing to identify or report financial transactions that involve the proceeds of cannabis-related violations of the Controlled Substances Act. We may also be exposed to the foregoing risks.
As previously introduced, in February 2014, FinCEN issued the FinCEN Memo providing instructions to banks seeking to provide services to cannabis-related businesses. The FinCEN Memo states that in some circumstances, it is permissible for banks to provide services to cannabis-related businesses without risking prosecution for violation of the Bank Secrecy Act. It refers to supplementary guidance that former Deputy Attorney General James M. Cole issued to federal prosecutors relating to the prosecution of money laundering offenses predicated on cannabis-related violations of the Controlled Substances Act. Although the FinCEN Memo remains in effect today, it is unclear at this time whether the current administration will follow the guidelines of the FinCEN Memo. Overall, the DOJ continues to have the right and power to prosecute crimes committed by banks and financial institutions, such as money laundering and violations of the Bank Secrecy Act, that occur in any state, including in states that have legalized the applicable conduct and the DOJ’s current enforcement priorities could change for any number of reasons. A change in the DOJ’s enforcement priorities could result in the DOJ prosecuting banks and financial institutions for crimes that previously were not prosecuted. If we do not have access to a U.S. banking system, its business and operations could be adversely affected.
Other potential violations of federal law resulting from cannabis-related activities include the Racketeer Influenced Corrupt Organizations Act (“RICO”). RICO is a federal statute providing criminal penalties in addition to a civil cause of action for acts performed as part of an ongoing criminal organization. Under RICO, it is unlawful for any person who has received income derived from a pattern of racketeering activity (which includes most felonious violations of the Canadian Securities Administrators), to use or invest any of that income in the acquisition of any interest, or the establishment or operation of, any enterprise which is engaged in interstate commerce. RICO also authorizes private parties whose properties or businesses are harmed by such patterns of racketeering activity to initiate a civil action against the individuals involved. Although RICO suits against the cannabis industry are rare, a few cannabis businesses have been subject to a civil RICO action. Defending such a case has proven extremely costly, and potentially fatal to a business’ operations.
In the event that any of our operations, or any proceeds thereof, any dividends or distributions therefrom, or any profits or revenues accruing from such operations in the United States were found to be in violation of money laundering legislation or otherwise, such transactions may be viewed as proceeds of crime under one or more of the statutes noted above or any other applicable legislation. This could restrict or otherwise jeopardize our ability to declare or pay dividends, effect other distributions or subsequently repatriate such funds back to Canada, and subject us to civil and/or criminal penalties. Furthermore, while there are no current intentions to declare or pay dividends on our Common Stock in the foreseeable future, in the event that a determination was made that our proceeds from operations (or any future operations or investments in the United States) could reasonably be shown to constitute proceeds of crime, we may decide or be required to suspend declaring or paying dividends without advance notice and for an indefinite period of time. We could likewise be required to suspend or cease operations entirely.
We may become subject to federal and state forfeiture laws which could negatively impact our business operations.
Violations of any federal laws and regulations could result in significant fines, penalties, administrative sanctions, convictions or settlements arising from civil proceedings conducted by either the federal government or private citizens, or criminal charges, including, but not limited to, seizure of assets, disgorgement of profits, cessation of business activities or divestiture. As an entity that conducts business in the cannabis industry, we are potentially subject to federal and state forfeiture laws (criminal and civil) that permit the government to seize the proceeds of criminal activity. Civil forfeiture laws could provide an alternative for the federal government or any state (or local police force) that wants to discourage residents from conducting transactions with cannabis related businesses but believes criminal liability is too difficult to prove beyond a reasonable doubt. Also, an individual can be required to forfeit property considered to be the proceeds of a crime even if the individual is not convicted of the crime, and the standard of proof in a civil forfeiture matter is lower than the standard in a criminal matter. Depending on the applicable law, whether federal or state, rather than having to establish liability beyond a reasonable doubt, the federal government or the state, as applicable, may be required to prove that the money or property at issue is proceeds of a crime only by either clear and convincing evidence or a mere preponderance of the evidence.
Investors located in states where cannabis remains illegal may be at risk of prosecution under federal and/or state conspiracy, aiding and abetting, and money laundering statutes, and be at further risk of losing their investments or proceeds under forfeiture statutes. Many states remain fully able to take action to prevent the proceeds of cannabis businesses from entering their state. Because state legalization is relatively new, it remains to be seen whether these states would take such action and whether a court would approve it. Investors and prospective investors of the Company should be aware of these potentially relevant federal and state laws in considering whether to invest in the Company.
We are subject to certain tax risks and treatments that could negatively impact our results of operations.
Section 280E of the Internal Revenue Code, as amended, prohibits businesses from deducting certain expenses associated with trafficking controlled substances (within the meaning of Schedule I and II of the Controlled Substances Act). The IRS has invoked Section 280E in tax audits against various cannabis businesses in the U.S. that are permitted under applicable state laws. Although the IRS issued a clarification allowing the deduction of certain expenses, the scope of such items is interpreted very narrowly and the bulk of operating costs and general administrative costs are not permitted to be deducted. While there are currently several pending cases before various administrative and federal courts challenging these restrictions, there is no guarantee that these courts will issue an interpretation of Section 280E favorable to cannabis businesses.
Our business in the cannabis industry is subject to heightened scrutiny by regulatory authorities.
For the reasons set forth above, our existing operations in the United States, and any future operations or investments, may become the subject of heightened scrutiny by regulators, stock exchanges and other authorities in Canada. As a result, we may be subject to significant direct and indirect interaction with public officials. There can be no assurance that this heightened scrutiny will not in turn lead to the imposition of certain restrictions on our ability to operate or invest in the United States or any other jurisdiction, in addition to those described herein.
Prior to the CDS MOU (as defined below), it had been reported by certain publications in Canada that The Canadian Depository for Securities Limited is considering a policy shift that would see its subsidiary, CDS Clearing and Depository Services Inc. (“CDS”), refuse to settle trades for cannabis issuers that have investments in the United States. CDS is Canada’s central securities depository, clearing and settlement hub settling trades in the Canadian equity, fixed income and money markets. CDS or its parent company has not issued any public statement in regard to these reports. If CDS were to proceed in the manner suggested by these publications, and apply such a policy to us, it would have a material adverse effect on the ability of holders of Common Stock to make trades in Canada. In particular, our Common Stock would become highly illiquid in Canada as investors would have no ability to effect a trade of our Common Stock in Canada through the facilities of a stock exchange.
In the United States, many clearing houses for major broker-dealer firms, including Pershing LLC, the largest clearing, custody and settlement firm in the United States, have refused to handle securities or settle transactions of companies engaged in cannabis related business. Many other clearing firms have taken a similar approach. This means that certain broker-dealers cannot accept for deposit or settle transactions in the securities of companies, which may inhibit the ability of investors to trade in our securities in the United States and could negatively affect the liquidity of our securities.
In addition, on November 24, 2017, the TMX Group provided an update regarding issuers with marijuana-related activities in the United States and confirmed that TMX Group will rely on the Canadian Securities Administrators’ recommendation to defer to individual exchange’s rules for companies that have marijuana-related activities in the United States and to determine the eligibility of individual issuers to list based on those exchanges’ listing requirements. On February 8, 2018, CDS signed a memorandum (the “CDS MOU”) with Aequitas NEO Exchange Inc., CNSX Markets Inc., TSX Inc., and TSX Venture Exchange Inc. (collectively, the “Exchanges”). The CDS MOU outlines CDS’ and the Exchanges’ understanding of Canada’s regulatory framework applicable to the rules and procedures and regulatory oversight of the Exchanges and CDS. The CDS MOU confirms, with respect to the clearing of listed securities, that CDS relies on the Exchanges to review the conduct of listed issuers. As a result, there currently is no CDS ban on the clearing of securities of issuers with marijuana-related activities in the U.S.
Any restrictions imposed by the CSE or other applicable exchange on the business of the Company and/or the potential delisting of our Common Stock from the CSE or other applicable exchange would have a material adverse effect on the Company and on the ability of holders of Common Stock to make trades in Canada.
The heightened regulatory scrutiny could have a negative impact on our ability to raise capital.
Our business activities rely on newly established and/or developing laws and regulations in multiple jurisdictions, including in Nevada. These laws and regulations are rapidly evolving and subject to change with minimal notice. Regulatory changes may adversely affect our profitability or cause it to cease operations entirely. The cannabis industry may come under the scrutiny or further scrutiny by the U.S. Food and Drug Administration, SEC, the DOJ, the Financial Industry Regulatory Authority or other federal, Nevada or other applicable state or non-governmental regulatory authorities or self-regulatory organizations that supervise or regulate the production, distribution, sale or use of cannabis for medical or non-medical purposes in the U.S. It is impossible to determine the extent of the impact of any new laws, regulations or initiatives that may be proposed, or whether any proposals will become law. The regulatory uncertainty surrounding our industry may adversely affect our business and operations, including without limitation, the costs to remain compliant with applicable laws and the impairment of its ability to raise additional capital, create a public trading market in the U.S. for securities of the Company or to find a suitable acquirer, which could reduce, delay or eliminate any return on investment in the Company.
Our business is subject to risk from changing regulatory and political environments surrounding the cannabis industry.
The success of our business strategy depends on the legality of the marijuana industry. The political environment surrounding the marijuana industry in general can be volatile and the regulatory framework remains in flux. To our knowledge, there are to date a total of 33 states, and the District of Columbia, Puerto Rico, the U.S. Virgin Islands and Guam that have legalized cannabis in some form, including Nevada, and additional states have pending legislation regarding the same; however, the risk remains that a shift in the regulatory or political realm could occur and have a drastic impact on the industry as a whole, adversely impacting our business, results of operations, financial condition or prospects.
Delays in enactment of new state or federal regulations could restrict our ability to reach strategic growth targets and lower return on investor capital. Our strategic growth strategy is reliant upon certain federal and state regulations being enacted to facilitate the legalization of medical and adult-use marijuana. If such regulations are not enacted, or enacted but subsequently repealed or amended, or enacted with prolonged phase-in periods, our growth target, and thus, the effect on the return of investor capital, could be detrimental. We are unable to predict with certainty when and how the outcome of these complex regulatory and legislative proceedings will affect its business and growth.
Further, there is no guaranty that state laws legalizing and regulating the sale and use of cannabis will not be repealed or overturned, or that local governmental authorities will not limit the applicability of state laws within their respective jurisdictions. If the federal government begins to enforce federal laws relating to cannabis in states where the sale and use of cannabis is currently legal, or if existing applicable state laws are repealed or curtailed, our business, results of operations, financial condition and prospects would be materially adversely affected. It is also important to note that local and city ordinances may strictly limit and/or restrict disbursement of marijuana in a manner that will make it extremely difficult or impossible to transact business that is necessary for the continued operation of the marijuana industry. Federal actions against individuals or entities engaged in the marijuana industry or a repeal of applicable marijuana related legislation could adversely affect us and our business, results of operations, financial condition and prospects.
We are aware that multiple states are considering special taxes or fees on businesses in the marijuana industry. It is a potential yet unknown risk at this time that other states are in the process of reviewing such additional fees and taxation. This could have a material adverse effect upon our business, results of operations, financial condition or prospects.
The commercial, medical and adult-use marijuana industries are in their infancy and we anticipate that such regulations will be subject to change as the jurisdictions in which we do business matures. We have in place a detailed compliance program overseen and maintained by external state and local regulatory/compliance counsel. Our internal compliance team (consisting of managers for each respective business unit) implements the compliance program.
Our internal compliance team oversees training for all employees, including on the following topics:
• compliance with state and local laws
• safe cannabis use
• dispensing procedures
• security and safety policies and procedures
• inventory control
• quality control
• transportation procedures
Our compliance program emphasizes security and inventory control to ensure strict monitoring of cannabis and inventory from delivery by a licensed distributor to sale or disposal. Only authorized, properly trained employees are allowed to access our computerized seed-to-sale system.
Additionally, we have created comprehensive standard operating procedures that include detailed descriptions and instructions for monitoring inventory at all stages of development and distribution. We will continue to monitor compliance on an ongoing basis in accordance with its compliance program, standard operating procedures, and any changes to regulation in the marijuana industry.
Overall, the medical and adult-use marijuana industry is subject to significant regulatory change at both the state and federal level. The inability of the Company to respond to the changing regulatory landscape may cause it to not be successful in capturing significant market share and could otherwise harm its business, results of operations, financial condition or prospects.
The potential re-classification of cannabis in the United States could create additional regulatory burdens on our operations and negatively affect our results of operations.
If cannabis and/or CBD is re-categorized as a Schedule II or lower controlled substance, the ability to conduct research on the medical benefits of cannabis would most likely be improved; however, rescheduling cannabis may materially alter enforcement policies across many federal agencies, primarily the U.S. Food and Drug Administration (the “FDA”). FDA is responsible for ensuring public health and safety through regulation of food, drugs, supplements, and cosmetics, among other products, through its enforcement authority pursuant to the Federal Food Drug and Cosmetic Act (the “FFDCA”). FDA’s responsibilities include regulating the ingredients as well as the marketing and labeling of drugs sold in interstate commerce. Because cannabis is federally illegal to produce and sell, and because it has no federally recognized medical uses, the FDA has historically deferred enforcement related to cannabis to the U.S. Drug Enforcement Agency (the “DEA”); however, the FDA has enforced the FFDCA with regard to hemp-derived products, especially CBD, sold outside of state-regulated cannabis businesses. If cannabis were to be rescheduled to a federally controlled, yet legal, substance, FDA would likely play a more active regulatory role. Further, in the event that the pharmaceutical industry directly competes with state-regulated cannabis businesses for market share, as could potentially occur with rescheduling, the pharmaceutical industry may urge the DEA, FDA, and others to enforce the Canadian Securities Administrators and FFDCA against businesses that comply with state but not federal law. The potential for multi-agency enforcement post-rescheduling could threaten or have a materially adverse effect on the operations of existing state-legal cannabis businesses, including the Company.
Even though certain U.S. and state statutes authorize the cultivation and transportation of CBD under certain circumstances, the DEA has determined that all CBD products, regardless of origin, are considered Schedule I controlled substances and issued a drug code for CBD. The United States Court of Appeals for the Ninth Circuit recently upheld the DEA’s rule. We are unable to determine whether this decision will have a chilling effect on sales of CBD products and whether our business will be adversely affected.
Our participation in the cannabis industry may lead to costly litigation, which could adversely affect our financial condition and business operations.
Our participation in the cannabis industry may lead to litigation, formal or informal complaints, enforcement actions, and inquiries by various federal, state, or local governmental authorities against us or our investments. Litigation, complaints, and enforcement actions involving either us or our investments could consume considerable amounts of financial and other corporate resources, which could have an adverse effect on our future cash flows, earnings, results of operations and financial condition.
There is uncertainty regarding the availability of U.S. federal patent and trademark protection.
As long as cannabis remains illegal under U.S. federal law, the benefit of certain federal laws and protections which may be available to most businesses, such as federal trademark and patent protection regarding the intellectual property of a business, may not be available to us. As a result, our intellectual property may never be adequately or sufficiently protected against the use or misappropriation by third-parties. In addition, since the regulatory framework of the cannabis industry is in a constant state of flux, we can provide no assurance that it will ever obtain any protection of its intellectual property, whether on a federal, state or local level.
Current constraints on marketing our products could adversely affect our sales and results of operations.
The development of our business and operating results may be hindered by applicable restrictions on sales and marketing activities imposed by government regulatory bodies. The regulatory environment in the United States limits companies’ abilities to compete for market share in a manner similar to other industries. If we are unable to effectively market our products and compete for market share, or if the costs of compliance with government legislation and regulation cannot be absorbed through increased selling prices for our products, our sales and results of operations could be adversely affected.
We could experience difficulty enforcing our contracts.
Due to the nature of our business and the fact that our contracts involve cannabis and other activities that are not legal under U.S. federal law and in some jurisdictions, we may face difficulties in enforcing our contracts in federal and certain state courts. The inability to enforce any of our contracts could have a material adverse effect on our business, operating results, financial condition or prospects.
Our payments system may depend on third-party providers and is subject to evolving laws and regulations.
We have engaged third-party service providers in the past, and may do so again in the future, to perform underlying debit card processing. If these service providers do not perform adequately our ability to process payments could be adversely affected and our business would be harmed.
The laws and regulations related to payments are complex and are potentially impacted by tensions between federal and state treatment of the cannabis industry. These laws and regulations also vary across different jurisdictions in which we operate. As a result, we are required to spend significant time and effort to comply with those laws and regulations. Any failure or claim of our failure to comply, or any failure by our third-party service providers to comply, could cost us substantial resources, could result in the failure of the third-party service provider to pay us, or could result in liabilities, which could have a material adverse effect on the Company.
Risks Related to the Business
We will require additional financing to support our on-going operations.
We will require equity and/or debt financing to support on-going operations, to undertake capital expenditures or to undertake acquisitions or other business combination transactions, such as the transactions contemplated by the IGH Option Agreement. A number of factors could cause us to incur higher borrowing costs and experience greater difficulty accessing public and private markets for debt. These factors include disruptions or declines in the global capital markets and/or a decline in our financial performance, outlook, or credit ratings. There can be no assurance that additional financing will be available to us when needed or on terms which are acceptable. Our inability to raise financing to fund on-going operations, capital expenditures or acquisitions may adversely affect our ability to fund our operations, meet contractual commitments, make future investments or desirable acquisitions, or respond to competitive challenges and may have a material adverse effect upon our business, results of operations, financial condition or prospects.
If additional funds are raised through further issuances of equity or convertible debt securities, existing shareholders could suffer significant dilution, and any new equity securities issued could have rights, preferences and privileges superior to those of holders of Common Stock. Any debt financing secured in the future could involve restrictive covenants relating to capital raising activities and other financial and operational matters, which may make it more difficult for us to obtain additional capital and to pursue business opportunities, including potential acquisitions.
We may have difficulty continuing as a going-concern.
The financial statements have been prepared on a going-concern basis under which an entity is considered to be able to realize its assets and satisfy its liabilities in the ordinary course of business. Our future operations are dependent upon the identification and successful completion of equity or debt financing and the achievement of profitable operations at an indeterminate time in the future. There can be no assurances that we will be successful in completing an equity or debt financing or in achieving profitability. The financial statements do not give effect to any adjustments relating to the carrying values and classification of assets and liabilities that would be necessary should we be unable to continue as a going-concern.
We had negative cash flow for the financial year ended May 31, 2019
We had negative operating cash flow for the financial year ended May 31, 2019. To the extent that we have negative operating cash flow in future periods, we may need to allocate a portion of our cash reserves to fund such negative cash flow. We may also be required to raise additional funds through the issuance of equity or debt securities. There can be no assurance that we will be able to generate a positive cash flow from our operations, that additional capital or other types of financing will be available when needed or that these financings will be on terms favorable to the Company.
We may experience difficulties in generating profits.
We may experience difficulties in our development process, such as capacity constraints, quality control problems or other disruptions, which would make it more difficult to generate profits. A failure by the Company to achieve a low-cost structure through economies of scale or improvements in manufacturing processes and design could have a material adverse effect on our business, prospects, results of operations and financial condition.
We will likely incur significant costs and obligations in relation to our on-going and anticipated business operations.
We expect to incur significant on-going costs and obligations related to our investment in infrastructure and growth and for regulatory compliance, which could have a material adverse impact on our results of operations, financial condition and cash flows. In addition, future changes in regulations, more vigorous enforcement thereof or other unanticipated events could require extensive changes to our operations, increased compliance costs or give rise to material liabilities, which could have a material adverse effect on the business, results of operations and financial condition of the Company.
Our business is reliant on Oasis and City Trees.
Our current activities and resources are focused on Oasis and City Trees. The licenses held by the Oasis LLCs are specific to Oasis and City Trees. Adverse changes or developments affecting any of Oasis or City Trees, including but not limited to, a breach of security, could have a material and adverse effect on our business, financial condition and prospects. Any breach of the security measures and other facility requirements, could also have an impact on the Oasis LLCs’ ability to continue operating under their respective licenses or the prospect of renewing their respective licenses. Oasis and City Trees continue to operate with routine maintenance however buildings do have components that require replacement. The Company will bear many, if not all, of the costs of maintenance and upkeep of Oasis and City Trees. Our operations and financial performance may be adversely affected if any of Oasis and City Trees are unable to keep up with maintenance requirements.
Furthermore, given our reliance on Oasis and City Trees, any negative publicity could have a material adverse effect on our business and operations, as could other regional occurrences such as local strikes, terrorist attacks, increases in energy prices, or natural or man-made disasters, or the enactment of more stringent state and local laws and regulations.
We are reliant on key employees in the management of our business and loss of their services could materially adversely affect our business.
Our success is dependent upon the ability, expertise, judgment, discretion and good faith of our senior management. While employment agreements or management agreements are customarily used as a primary method of retaining the services of key employees, these agreements cannot assure the continued services of such employees. Any loss of the services of such individuals could have a material adverse effect on our business, operating results, financial condition or prospects.
Our business is heavily regulated which could have a material adverse effect on our results of operations and financial condition.
The business and activities of the Company are heavily regulated in all jurisdictions where it carries on business. Our operations are subject to various laws, regulations and guidelines by governmental authorities, relating to the manufacture, marketing, management, transportation, storage, sale, pricing and disposal of medical marijuana and cannabis oil, and also including laws and regulations relating to health and safety, insurance coverage, the conduct of operations and the protection of the environment. Laws and regulations, applied generally, grant government agencies and self-regulatory bodies broad administrative discretion over the activities of the Company, including the power to limit or restrict business activities as well as impose additional disclosure requirements on our products and services. Achievement of our business objectives is contingent, in part, upon compliance with regulatory requirements enacted by governmental authorities and obtaining all regulatory approvals, where necessary, for the sale of our products. Similarly, the Company cannot predict the time required to secure all appropriate regulatory approvals for its products, or the extent of testing and documentation that may be required by governmental authorities. Any delays in obtaining, or failure to obtain regulatory approvals would significantly delay the development of markets and products and could have a material adverse effect on the business, results of operations and financial condition of the Company.
We will incur ongoing costs and obligations related to regulatory compliance. Failure to comply with regulations may lead to possible sanctions including the revocation or imposition of additional conditions on licenses to operate our business, the suspension or expulsion from a particular market or jurisdiction or of our key personnel, and the imposition of fines and censures. In addition, changes in regulations, more vigorous enforcement thereof or other unanticipated events could require extensive changes to our operations, increased compliance costs or give rise to material liabilities, which could have a material adverse effect on the business, results of operations and financial condition of the Company.
Our business is subject to general regulatory risks, which could negatively impact our operations.
Our business is subject to a variety of laws, regulations and guidelines relating to the manufacture, management, transportation, storage and disposal of marijuana, including laws and regulations relating to health and safety, the conduct of operations and the protection of the environment. Achievement of our business objectives are contingent, in part, upon compliance with applicable regulatory requirements and obtaining all requisite regulatory approvals. Changes to such laws, regulations and guidelines due to matters beyond the control of the Company may cause adverse effects to the Company.
We are required to obtain or renew further government permits and licenses for our current and contemplated operations. Obtaining, amending or renewing the necessary governmental permits and licenses can be a time-consuming process potentially involving numerous regulatory agencies, involving public hearings and costly undertakings on our part. The duration and success of our efforts to obtain, amend and renew permits and licenses are contingent upon many variables not within our control, including the interpretation of applicable requirements implemented by the relevant permitting or licensing authority. We may not be able to obtain, amend or renew permits or licenses that are necessary to our operations. Any unexpected delays or costs associated with the permitting and licensing process could impede the ongoing or proposed operations of the Company. To the extent permits or licenses are not obtained, amended or renewed, or are subsequently suspended or revoked, the Company may be curtailed or prohibited from proceeding with its ongoing operations or planned development and commercialization activities. Such curtailment or prohibition may result in a material adverse effect on our business, financial condition, results of operations or prospects.
While our compliance controls have been developed to mitigate the risk of any material violations of any license we hold, there is no assurance that our licenses will be renewed by each applicable regulatory authority in the future in a timely manner. Any unexpected delays or costs associated with the licensing renewal process for any of the licenses held by the Company could impede the ongoing or planned operations of the Company and have a material adverse effect on our business, financial condition, results of operations or prospects.
We may become involved in a number of government or agency proceedings, investigations and audits. The outcome of any regulatory or agency proceedings, investigations, audits, and other contingencies could harm our reputation, require the Company to take, or refrain from taking, actions that could harm its operations or require the Company to pay substantial amounts of money, harming its financial condition. There can be no assurance that any pending or future regulatory or agency proceedings, investigations and audits will not result in substantial costs or a diversion of management’s attention and resources or have a material adverse impact on our business, financial condition, results of operations or prospects.
Changes in laws, regulations and guidelines could have a material adverse effect on the business, results of operations and financial condition of the Company.
Our operations are subject to various laws, regulations, guidelines and licensing requirements relating to the production, manufacture, sale, distribution, management, transportation, storage and disposal of medical marijuana, as well as being subject to laws and regulations relating to health and safety, the conduct of operations and the protection of the environment. While to the knowledge of management we are currently in compliance with all such state laws, any changes to such laws, regulations, guidelines and policies due to matters beyond the control of the Company could have a material adverse effect on the business, results of operations and financial condition of the Company.
Volatility of industry conditions could have a material adverse effect on our operations.
Industry conditions are influenced by numerous factors over which we have no control, including the level of medical marijuana prices, expectations about future medical marijuana prices and production, the cost of producing and delivering medical marijuana; any rates of declining current production, political, regulatory and economic conditions; alternative fuel requirements; and the ability of medical marijuana companies to raise equity capital or debt financing.
The level of activity in the medical marijuana industry is volatile. No assurance can be given that expected trends in medical marijuana production and sales activities will continue or that demand for medical marijuana will reflect the level of activity in the industry. Any prolonged substantial reduction in medical marijuana prices would likely affect medical marijuana production levels and therefore affect the demand for medical marijuana. A material decline in medical marijuana prices or industry activity could have a material adverse effect on our business, financial condition, results of operations and cash flows.
Our industry is subject to intense competition.
There is potential that the Company will face intense competition from other companies, some of which can be expected to have longer operating histories and more financial resources and experience than the Company. Increased competition by larger and better-financed competitors could materially and adversely affect the business, financial condition, results of operations or prospects of the Company. If we are unable to compete effectively, it could decrease our customer traffic, sales and profit margins, which could adversely affect our business, financial condition, and results of operations.
Because of the early stage of the industry in which the Company operates, the Company expects to face additional competition from new entrants. To become and remain competitive, the Company will require research and development, marketing, sales and support. We may not have sufficient resources to maintain research and development, marketing, sales and support efforts on a competitive basis which could materially and adversely affect the business, financial condition, results of operations or prospects of the Company.
The introduction of a recreational model for cannabis production and distribution may impact the medical marijuana market. The impact of this potential development may be negative for the Company, and could result in increased levels of competition in its existing medical market and/or the entry of new competitors in the overall cannabis market in which the Company operates.
If the number of users of medical marijuana increases, the demand for products will increase and the Company expects that competition will become more intense, as current and future competitors begin to offer an increasing number of diversified products. To remain competitive, the Company will require a continued high level of investment in research and development, marketing, sales and client support. We may not have sufficient resources to maintain research and development, marketing, sales and client support efforts on a competitive basis which could materially and adversely affect the business, financial condition and results of operations of the Company.
As well, the legal landscape for medical and recreational marijuana is changing internationally. More countries have passed laws that allow for the production and distribution of medical marijuana in some form or another. We have some international partnerships in place, which may be effected if more countries legalize medical marijuana. Increased international competition might lower the demand for our products on a global scale.
New well-capitalized entrants into our industry may develop large-scale operations which will make it difficult for our business to compete and remain profitable.
Currently, the marijuana industry generally is comprised of individuals and small to medium-sized entities, however, the risk remains that large conglomerates and companies who also recognize the potential for financial success through investment in this industry could strategically purchase or assume control of larger dispensaries and cultivation facilities. In doing so, these larger competitors could establish price setting and cost controls which would effectively “price out” many of the individuals and small to medium-sized entities who currently make up the bulk of the participants in the varied businesses operating within and in support of the medical marijuana industry. While the trend in most state laws and regulations seemingly deters this type of takeover, this industry remains quite nascent, so what the landscape will be in the future remains largely unknown, which in itself is a risk.
Our proposed business plan is subject to all business risks associated with new business enterprises, including the absence of any significant operating history upon which to evaluate an investment. The likelihood of our success must be considered in light of the problems, expenses, difficulties, complications and delays frequently encountered in connection with the formation of a new business, the development of new strategy and the competitive environment in which the Company will operate. It is possible that the Company will incur losses in the future. There is no guarantee that the Company will be profitable.
We could incur risks and uncertainties regarding our future acquisitions and dispositions.
Material acquisitions, dispositions and other strategic transactions, including the transactions contemplated by the IGH Option Agreement, involve a number of risks, including: (i) potential disruption of our ongoing business; (ii) distraction of management; (iii) the Company may become more financially leveraged; (iv) the anticipated benefits and cost savings of those transactions may not be realized fully or at all or may take longer to realize than expected; (v) increasing the scope and complexity of our operations; and (vi) loss or reduction of control over certain of our assets.
The presence of one or more material liabilities of an acquired company that are unknown to us at the time of acquisition could have a material adverse effect on the business, results of operations, prospects and financial condition of the Company. A strategic transaction may result in a significant change in the nature of our business, operations and strategy. In addition, the Company may encounter unforeseen obstacles or costs in implementing a strategic transaction or integrating any acquired business into our operations.
Acquisitions and strategic collaborations may never materialize or may fail.
We intend to explore a variety of acquisitions and strategic collaborations with existing marijuana growers, dispensaries and related businesses in various states, such as the transactions contemplated by the IGH Option Agreement. We are likely to face significant competition in seeking appropriate acquisitions or strategic collaborators, and these acquisitions and strategic collaborations can be complicated and time consuming to negotiate and document. We may not be able to negotiate acquisitions and strategic collaborations on acceptable terms, or at all, and we are unable to predict when, if ever, we will enter into any such acquisitions or strategic collaborations due to the numerous risks and uncertainties associated with them.
Failure to successfully integrate acquired businesses, their products and other assets into the Company, or if integrated, failure to further our business strategy, may result in our inability to realize any benefit from such acquisition.
We have grown by acquiring Alternative Solutions. The consummation and integration of any acquired business, product or other assets into the Company may be complex and time consuming and, if Alternative Solutions and its assets are not successfully integrated, the Company may not achieve the anticipated benefits, cost-savings or growth opportunities. Furthermore, the Alternative Solutions acquisition and other arrangements, even if successfully integrated, may fail to further the Company’s business strategy as anticipated, expose the Company to increased competition or other challenges with respect to the Company’s products or geographic markets, and expose the Company to additional liabilities associated with an acquired business, technology or other asset or arrangement.
When the Company acquires cannabis businesses, it may obtain the rights to applications for licenses as well as licenses; however, the procurement of such applications for licenses and licenses generally will be subject to governmental and regulatory approval. There are no guarantees that the Company will successfully consummate such acquisitions, and even if the Company consummates such acquisitions, the procurement of applications for licenses may never result in the grant of a license by any state or local governmental or regulatory agency and the transfer of any rights to licenses may never be approved by the applicable state and/or local governmental or regulatory agency.
Investors will have limited recourse against sellers of Alternative Solutions.
Investors in the Company will not have a direct statutory right or any other rights against the sellers of Alternative Solutions. The sole remedy of the investors against the sellers of Alternative Solutions will be through the Company bringing an action for a breach of the representations and warranties contained in the Acquisition Agreement. While the Company generally is indemnified for breaches of representations and warranties contained in the Acquisition Agreement, recourse for such breaches may be limited due to qualifications related to knowledge of the sellers or otherwise, contractual and time limits on recourse under applicable laws, and the ability of the vendors to satisfy third-party claims. In particular, most of the representations and warranties under the Acquisition Agreement survived for a period of only one year and have now expired. The inability to recover fully any significant liabilities incurred with respect to breaches of representations and warranties under the Acquisition Agreement may have adverse effects on our financial position. In addition, the sellers did not make any representation to the Company, and have not made any representation to investors, as to the disclosure in this Annual Report on Form 10-K constituting full, true and plain disclosure of all material facts related to the Acquisition, or that this Annual Report on Form 10-K does not contain a misrepresentation with respect to such Acquisition. Accordingly, the sellers will not have any liability to investors if the disclosure in this Annual Report on Form 10-K relating to the Acquisition does not meet such standard or contains a misrepresentation.
The Company is a holding company.
The Company is a holding company and essentially all of its assets are the capital stock of its material subsidiaries. As a result, investors in the Company are subject to the risks attributable to its subsidiaries. Consequently, our cash flows and ability to complete current or desirable future enhancement opportunities are dependent on the earnings of its subsidiaries and investments and the distribution of those earnings to the Company. The ability of these entities to pay dividends and other distributions will depend on their operating results and will be subject to applicable laws and regulations which require that solvency and capital standards be maintained by such companies and contractual restrictions contained in the instruments governing their debt. In the event of a bankruptcy, liquidation or reorganization of any of the Company’s material subsidiaries, holders of indebtedness and trade creditors may be entitled to payment of their claims from the assets of those subsidiaries before the Company.
We have a limited operating history.
The Company and its subsidiaries have varying and limited operating histories, which can make it difficult for investors to evaluate our operations and prospects and may increase the risks associated with investment into the Company.
We have not generated profits in the periods covered by our financial statements included herein, and, as a result, have only a very limited operating history upon which our business and future prospects may be evaluated.
Although the Company expects to generate substantial revenues from its subsidiaries, the subsidiaries have only started generating revenues during the last fiscal year and accordingly, we are therefore expected to remain subject to many of the risks common to early-stage enterprises for the foreseeable future, including challenges related to laws, regulations, licensing, integrating and retaining qualified employees; making effective use of limited resources; achieving market acceptance of existing and future solutions; competing against companies with greater financial and technical resources; acquiring and retaining customers; and developing new solutions. There is no assurance that the Company will be successful in achieving a return on shareholders’ investment and the likelihood of success must be considered in light of the early stage of operations.
Potential reputational risks to third parties could result in difficulties in maintaining our operations.
The parties with which the Company does business may perceive that they are exposed to reputational risk as a result of our medical marijuana business activities. While we have other banking relationships and believe that the services can be procured from other institutions, the Company may in the future have difficulty establishing or maintaining bank accounts or other business relationships. Failure to establish or maintain business relationships could have a material adverse effect on the Company.
Changes in public opinion and perception could negatively affect our business operations.
Government policy changes or public opinion may also result in a significant influence over the regulation of the cannabis industry in the United States or elsewhere. Public opinion and support for medical and adult-use marijuana has traditionally been inconsistent and varies from jurisdiction to jurisdiction. While public opinion and support appears to be rising for legalizing medical and adult-use marijuana, it remains a controversial issue subject to differing opinions surrounding the level of legalization (for example, medical marijuana as opposed to legalization in general). A negative shift in the public’s perception of cannabis in the United States or any other applicable jurisdiction could affect future legislation or regulation. Among other things, such a shift could cause state jurisdictions to abandon initiatives or proposals to legalize medical and/or adult-use cannabis, thereby limiting the number of new state jurisdictions into which the Company could expand. Any inability to fully implement our expansion strategy may have a material adverse effect on its business, results of operations or prospects.
We may be subject to unfavorable publicity or consumer perception which could negatively affect our results of operations.
We believe the medical marijuana industry is highly dependent upon consumer perception regarding the safety, efficacy and quality of the marijuana produced. Consumer perception can be significantly influenced by scientific research or findings, regulatory investigations, litigation, media attention and other publicity regarding the consumption of marijuana products. There can be no assurance that future scientific research or findings, regulatory investigations, litigation, media attention or other publicity will be favorable to the marijuana market or any particular product, or consistent with earlier publicity. Future research reports, findings, regulatory investigations, litigation, media attention or other publicity that are perceived as less favorable than, or that question, earlier research reports, findings or other publicity could have a material adverse effect on the demand for medical marijuana and on the business, results of operations, financial condition, cash flows or prospects of the Company. Further, adverse publicity reports or other media attention regarding the safety, efficacy and quality of marijuana in general, or associating the consumption of medical marijuana with illness or other negative effects or events, could have such a material adverse effect. There is no assurance that such adverse publicity reports or other media attention will not arise.
Research and development costs may negatively impact our results of operations.
Before the Company can obtain regulatory approval for the commercial sale of any of its products, it will be required to complete extensive trial testing to demonstrate safety and efficacy. Depending on the exact nature of trial testing, such trials can be expensive and are difficult to design and implement. The testing process is also time consuming and can often be subject to unexpected delays.
The timing and completion of trial testing may be subject to significant delays relating to various causes, including: inability to manufacture or obtain sufficient quantities of units and or test subjects for use in trial testing; delays arising from collaborative partnerships; delays in obtaining regulatory approvals to commence a study, or government intervention to suspend or terminate a study; delays, suspensions or termination of trial testing due to the applicable institutional review board or independent ethics board responsible for overseeing the study to protect research subjects; delays in identifying and reaching agreement on acceptable terms with prospective trial testing sites and subjects; variability in the number and types of subjects available for each study and resulting difficulties in identifying and enrolling subjects who meet trial eligibility criteria; scheduling conflicts; difficulty in maintaining contact with subjects after testing, resulting in incomplete data; unforeseen safety issues or side effects; lack of efficacy during trial testing; reliance on research organizations to conduct trial testing, which may not conduct such trials with good laboratory practices; or other regulatory delays.
We may experience difficulty in developing products.
If the Company cannot successfully develop, manufacture and distribute its products, or if the Company experiences difficulties in the development process, such as capacity constraints, quality control problems or other disruptions, the Company may not be able to develop market-ready commercial products at acceptable costs, which would adversely affect our ability to effectively enter the market. A failure by the Company to achieve a low-cost structure through economies of scale or improvements in cultivation and manufacturing processes would have a material adverse effect on our commercialization plans and our business, prospects, results of operations and financial condition.
We are dependent on the success of our new and existing products and services.
We have committed, and expect to continue to commit, significant resources and capital to develop and market existing product and service enhancements and new products and services. These products and services are relatively untested, and the Company cannot guarantee that it will achieve market acceptance for these products and services, or other new products and services that we may offer in the future. Moreover, these and other new products and services may be subject to significant competition with offerings by new and existing competitors in the business of manufacturing and distributing vaporizers and accessories. In addition, new products, services and enhancements may pose a variety of technical challenges and require us to attract additional qualified employees. The failure to successfully develop and market these new products, services or enhancements or to hire qualified employees could seriously harm our business, financial condition and results of operations.
We are dependent on the continued market acceptance by consumers of our products.
We are substantially dependent on continued market acceptance of our products by consumers. Although we believe that the use of products similar to the products designed and manufactured by the Company is gaining international acceptance, we cannot predict the future growth rate and size of this market.
We may incur significant expenses in promoting and maintaining brands, which could negatively impact our profitability.
We believe that establishing and maintaining the brand identities of products is a critical aspect of attracting and expanding a large customer base. Promotion and enhancement of brands will depend largely on success in continuing to provide high quality products. If customers and end users do not perceive our products to be of high quality, or if the Company introduces new products or enters into new business ventures that are not favorably received by customers and end users, the Company will risk diluting brand identities and decreasing their attractiveness to existing and potential customers. Moreover, in order to attract and retain customers and to promote and maintain brand equity in response to competitive pressures, the Company may have to increase substantially financial commitment to creating and maintaining a distinct brand loyalty among customers. If the Company incurs significant expenses in an attempt to promote and maintain brands, the business, results of operations and financial condition could be adversely affected.
The results of future clinical research may negatively impact our business.
Research in Canada, the U.S. and internationally regarding the medical benefits, viability, safety, efficacy, dosing and social acceptance of cannabis or isolated cannabinoids (such as CBD and THC) remains in early stages. There have been relatively few clinical trials on the benefits of cannabis or isolated cannabinoids (such as CBD and THC). Although the Company believes that the articles, reports and studies support its beliefs regarding the medical benefits, viability, safety, efficacy, dosing and social acceptance of cannabis, future research and clinical trials may prove such statements to be incorrect, or could raise concerns regarding, and perceptions relating to, cannabis. Given these risks, uncertainties and assumptions, prospective purchasers of our Common Stock should not place undue reliance on such articles and reports. Future research studies and clinical trials may draw opposing conclusions to those stated in this Prospectus or reach negative conclusions regarding the medical benefits, viability, safety, efficacy, dosing, social acceptance or other facts and perceptions related to cannabis, which could have a material adverse effect on the demand for our products with the potential to lead to a material adverse effect on our business, financial condition, results of operations or prospects.
We are reliant on key inputs and changes in their costs could negatively impact our profitability.
The manufacturing business is dependent on a number of key inputs and their related costs including raw materials and supplies related to product development and manufacturing operations. Any significant interruption or negative change in the availability or economics of the supply chain for key inputs could materially impact the business, financial condition, results of operations or prospects of the Company. Some of these inputs may only be available from a single supplier or a limited group of suppliers. If a sole source supplier was to go out of business, the Company might be unable to find a replacement for such source in a timely manner or at all. If a sole source supplier were to be acquired by a competitor, that competitor may elect not to sell to the Company in the future. Any inability to secure required supplies and services or to do so on appropriate terms could have a materially adverse impact on the business, financial condition, results of operations or prospects of the Company.
We are subject to environmental regulations.
Our operations are subject to environmental regulation in the various jurisdictions in which we operate. These regulations mandate, among other things, the maintenance of air and water quality standards and land reclamation. They also set forth limitations on the generation, transportation, storage and disposal of solid and hazardous waste. Environmental legislation is evolving in a manner which will require stricter standards and enforcement, increased fines and penalties for non-compliance, more stringent environmental assessments of proposed projects and a heightened degree of responsibility for companies and their officers, directors and employees. There is no assurance that future changes in environmental regulation, if any, will not adversely affect our operations.
Government environmental approvals and permits are currently, and may in the future be required in connection with CLSH’s operations. To the extent such approvals are required and not obtained, the Company may be curtailed or prohibited from its proposed business activities or from proceeding with the development of its operations as currently proposed.
Failure to comply with applicable environmental laws, regulations and permitting requirements may result in enforcement actions thereunder, including orders issued by regulatory or judicial authorities causing operations to cease or be curtailed, and may include corrective measures requiring capital expenditures, installation of additional equipment, or remedial actions. We may be required to compensate those suffering loss or damage due to our operations and may have civil or criminal fines or penalties imposed for violations of applicable laws or regulations.
Our business is subject to certain environmental risks.
Our operations are subject to environmental regulation in the various jurisdictions in which we operate. These regulations mandate, among other things, the maintenance of air and water quality standards and land reclamation. They also set forth limitations on the generation, transportation, storage and disposal of solid and hazardous waste. Environmental legislation is evolving in a manner which will require stricter standards and enforcement, increased fines and penalties for non-compliance, more stringent environmental assessments of proposed projects and a heightened degree of responsibility for companies and their officers, directors (or the equivalent thereof) and employees. There is no assurance that future changes in environmental regulation, if any, will not adversely affect our operations.
Government approvals and permits are currently, and may in the future, be required in connection with our operations. To the extent such approvals are required and not obtained, the Company may be curtailed or prohibited from its proposed production of medical marijuana or from proceeding with the development of its operations as currently proposed.
Failure to comply with applicable laws, regulations and permitting requirements may result in enforcement actions thereunder, including orders issued by regulatory or judicial authorities causing operations to cease or be curtailed, and may include corrective measures requiring capital expenditures, installation of additional equipment, or remedial actions. We may be required to compensate those suffering loss or damage by reason of our operations and may have civil or criminal fines or penalties imposed for violations of applicable laws or regulations.
Amendments to current laws, regulations and permits governing the production of medical marijuana, or more stringent implementation thereof, could have a material adverse impact on the Company and cause increases in expenses, capital expenditures or production costs or reduction in levels of production or require abandonment or delays in development.
Our business is subject to certain agricultural risks.
Our future business involves the growing of cannabis, an agricultural product. Such business will be subject to the risks inherent in the agricultural business, such as insects, plant diseases and similar agricultural risks. Although the Company expects that any such growing will be completed indoors under climate controlled conditions, there can be no assurance that natural elements will not have a material adverse effect on any such future production.
Our business is vulnerable to rising energy costs.
Adult-use and medical marijuana growing operations consume considerable energy, making the Company potentially vulnerable to rising energy costs. Rising or volatile energy costs may adversely impact the business, results of operations, financial condition or prospects of the Company.
We are dependent on equipment and skilled labor.
Our ability to compete and grow is dependent on our having access, at a reasonable cost and in a timely manner, to skilled labor, equipment, parts and components. No assurances can be given that we will be successful in maintaining our required supply of skilled labor, equipment, parts and components. It is also possible that the final costs of the major equipment contemplated by our capital expenditure plans may be significantly greater than anticipated by our management, and may be greater than funds available to us, in which circumstance the Company may curtail, or extend the timeframes for completing, its capital expenditure plans. This could have an adverse effect on the business, financial condition, results of operations or prospects of the Company.
The market for our products is difficult to forecast and our forecasts may not be accurate which could negatively impact our results of operations.
We must rely largely on our own market research to forecast sales as detailed forecasts are not generally obtainable from other sources at this early stage of the industry. A failure in the demand for our products to materialize as a result of competition, technological change or other factors could have a material adverse effect on the business, results of operations, financial condition or prospects of the Company.
We are subject to certain risks regarding the management of our growth.
We may be subject to growth-related risks including capacity constraints and pressure on our internal systems and controls. The ability of the Company to manage growth effectively will require it to continue to implement and improve its operational and financial systems and to expand, train and manage its employee base. The inability of the Company to deal with this growth may have a material adverse effect on our business, financial condition, results of operations or prospects.
We may experience difficulties in maintaining adequate internal controls.
Effective internal controls are necessary for the Company to provide reliable financial reports and to help prevent fraud. Failure to implement required new or improved controls, or difficulties encountered in their implementation, could harm our results of operations or cause it to fail to meet its reporting obligations. If the Company or its auditors discover a material weakness, the disclosure of that fact, even if quickly remedied, could reduce the market’s confidence in our Consolidated Financial Statements and materially adversely affect the trading price of our Common Stock.
Certain of our officers and directors may have conflicts of interest.
Certain of the directors and officers of the Company are, or may become directors and officers of other companies, and conflicts of interest may arise between their duties as officers and directors of the Company and as officers and directors of such other companies.
We may become subject to costly litigation regarding our operations.
We may become party to litigation from time to time in the ordinary course of business which could adversely affect our business. Should any litigation in which the Company becomes involved be determined against the Company, such a decision could adversely affect our ability to continue operating and the market price for our Common Stock. Even if we are involved in litigation and win, litigation can redirect significant company resources.
We are subject to product liability regarding our products, which could result in costly litigation and settlements.
As a distributor of products designed to be ingested by humans, the Company faces an inherent risk of exposure to product liability claims, regulatory action and litigation if its products are alleged to have caused significant loss or injury. In addition, the sale of our products involves the risk of injury to consumers due to tampering by unauthorized third parties or product contamination. Previously unknown adverse reactions resulting from human consumption of our products alone or in combination with other medications or substances could occur. We may be subject to various product liability claims, including, among others, that our products caused injury or illness, include inadequate instructions for use or include inadequate warnings concerning possible side effects or interactions with other substances.
A product liability claim or regulatory action against the Company could result in increased costs, could adversely affect our reputation with our clients and consumers generally, and could have a material adverse effect on our results of operations and financial condition of the Company. Although we have secured product liability insurance, and strictly enforce a quality standard within the operations, there can be no assurances that we will be able to maintain our product liability insurance on acceptable terms or with adequate coverage against potential liabilities. This scenario could prevent or inhibit the commercialization of our potential products. To date, there have been no product related issues.
Our products may become subject to product recalls, which could negatively impact our results of operations.
Manufacturers and distributors of products are sometimes subject to the recall or return of their products for a variety of reasons, including product defects, such as contamination, unintended harmful side effects or interactions with other substances, packaging safety and inadequate or inaccurate labeling disclosure. If any of our products are recalled due to an alleged product defect or for any other reason, the Company could be required to incur the unexpected expense of the recall and any legal proceedings that might arise in connection with the recall. We may lose a significant amount of sales and may not be able to replace those sales at an acceptable margin or at all. In addition, a product recall may require significant management attention. Although we have detailed procedures in place for testing finished products, there can be no assurance that any quality, potency or contamination problems will be detected in time to avoid unforeseen product recalls, regulatory action or lawsuits. Additionally, if one of our significant brands were subject to recall, the image of that brand and the Company as its owner could be harmed. A recall for any of the foregoing reasons could lead to decreased demand for the Company’s products and could have a material adverse effect on the results of operations and financial condition of the Company. Additionally, product recalls may lead to increased scrutiny of our operations by the U.S. FDA, Health Canada or other regulatory agencies, requiring further management attention and potential legal fees and other expenses.
We are subject to certain intellectual property risks.
Our viability will depend, in part, on our ability to develop and maintain the proprietary aspects of our technology to distinguish our products from our competitors’ products. We have certain proprietary intellectual property, including but not limited to brands, trademarks, trade names, patents and proprietary processes. We will rely on this intellectual property, know-how and other proprietary information, and may require employees, consultants and suppliers to sign confidentiality agreements. However, any confidentiality agreement may be breached, and the Company may not have adequate remedies for such breaches. Third parties may independently develop substantially equivalent proprietary information without infringing upon any proprietary technology. Third parties may otherwise gain access to our proprietary information and adopt it in a competitive manner. Any loss of intellectual property protection may have a material adverse effect on our business, results of operations or prospects.
As long as cannabis remains illegal under U.S. federal law as a Schedule I controlled substance pursuant to the Controlled Substances Act, the benefit of certain federal laws and protections which may be available to most businesses, such as federal trademark and patent protection regarding the intellectual property of a business, may not be available us. As a result, our intellectual property may never be adequately or sufficiently protected against the use or misappropriation by third parties. In addition, since the regulatory framework of the cannabis industry is in a constant state of flux, the Company can provide no assurance that it will ever obtain any protection of its intellectual property, whether on a federal, state, provincial and/ or local level.
We may also find it necessary to bring infringement or other actions against third parties to seek to protect its intellectual property rights. Litigation of this nature, even if successful, is often expensive and time-consuming to prosecute and there can be no assurance that we will have the financial or other resources to enforce our rights or prevent other parties from developing similar technology or designing around our intellectual property. Although we believe that our technology does not and will not infringe upon the patents or violate the proprietary rights of others, it is possible such infringement or violation has occurred or may occur, which could have a material adverse effect on our business.
We are not aware of any infringement by us of any person’s or entity’s intellectual property rights. In the event that products the Company sells are deemed to infringe upon the patents or proprietary rights of others, the Company could be required to modify its products or obtain a license for the manufacture and/or sale of such products or cease selling such products. In such event, there can be no assurance that the Company would be able to do so in a timely manner, upon acceptable terms and conditions, or at all, and the failure to do any of the foregoing could have a material adverse effect upon our business.
There can be no assurance that the Company will have the financial or other resources necessary to enforce or defend a patent infringement or proprietary rights violation action. If our products or proposed products are deemed to infringe or likely to infringe upon the patents or proprietary rights of others, the Company could be subject to injunctive relief and, under certain circumstances, become liable for damages, which could also have a material adverse effect on our business and financial condition.
Fraudulent or illegal activity by employees, contractors and consultants could negatively impact our operations.
We are exposed to the risk that our employees, independent contractors and consultants may engage in fraudulent or other illegal activity. Misconduct by these parties could include intentional, reckless and/or negligent conduct or disclosure of unauthorized activities to us that violates: (i) government regulations; (ii) manufacturing standards; (iii) federal and provincial healthcare fraud and abuse laws and regulations; or (iv) laws that require the true, complete and accurate reporting of financial information or data. It may not always be possible for the Company to identify and deter misconduct by its employees and other third parties, and the precautions taken by the Company to detect and prevent this activity may not be effective in controlling unknown or unmanaged risks or losses or in protecting the Company from governmental investigations or other actions or lawsuits stemming from a failure to be in compliance with such laws or regulations. If any such actions are instituted against the Company, and it is not successful in defending itself or asserting its rights, those actions could have a significant impact on our business, including the imposition of civil, criminal and administrative penalties, damages, monetary fines, contractual damages, reputational harm, diminished profits and future earnings, and curtailment of our operations, any of which could have a material adverse effect on our business, financial condition, results of operations or prospects.
We are subject to certain risks regarding our information technology systems and cyber-attacks.
Our operations depend, in part, on how well we and our suppliers protect networks, equipment, IT systems and software against damage from a number of threats, including, but not limited to, cable cuts, damage to physical plants, natural disasters, intentional damage and destruction, fire, power loss, hacking, computer viruses, vandalism and theft. Our operations also depend on the timely maintenance, upgrade and replacement of networks, equipment, IT systems and software, as well as pre-emptive expenses to mitigate the risks of failures. Any of these and other events could result in information system failures, delays and/or increase in capital expenses. The failure of information systems or a component of information systems could, depending on the nature of any such failure, adversely impact our reputation and results of operations.
We have not experienced any material losses to date relating to cyber-attacks or other information security breaches, but there can be no assurance that the Company will not incur such losses in the future. Our risk and exposure to these matters cannot be fully mitigated because of, among other things, the evolving nature of these threats. As a result, cyber security and the continued development and enhancement of controls, processes and practices designed to protect systems, computers, software, data and networks from attack, damage or unauthorized access is a priority. As cyber threats continue to evolve, the Company may be required to expend additional resources to continue to modify or enhance protective measures or to investigate and remediate any security vulnerabilities.
If we experience security breaches, it could negatively impact our operations and result in litigation or civil penalties and fees.
Given the nature of our product and its lack of legal availability outside of channels approved by the Government of the United States, as well as the concentration of inventory in its facilities, despite meeting or exceeding all legislative security requirements, there remains a risk of shrinkage as well as theft. A security breach at one of our facilities could expose the Company to additional liability and to potentially costly litigation, increase expenses relating to the resolution and future prevention of these breaches and may deter potential patients from choosing our products.
In addition, the Company collects and stores personal information about its customers and is responsible for protecting that information from privacy breaches. A privacy breach may occur through procedural or process failure, information technology malfunction, or deliberate unauthorized intrusions. Theft of data for competitive purposes, particularly patient lists and preferences, is an ongoing risk whether perpetrated via employee collusion or negligence or through deliberate cyber-attack. Any such theft or privacy breach would have a material adverse effect on our business, financial condition and results of operations.
We are subject to market price volatility risks.
The market price of our Common Stock may be subject to wide fluctuations in response to many factors, including variations in the operating results of the Company, divergence in financial results from analysts’ expectations, changes in earnings estimates by stock market analysts, changes in the business prospects for the Company, general economic conditions, legislative changes, and other events and factors outside of our control. In addition, stock markets have from time to time experienced extreme price and volume fluctuations, which, as well as general economic and political conditions, could adversely affect the market price for our Common Stock.
The lack of reliable data on the medical marijuana industry may negatively impact our results of operations.
As a result of recent and ongoing regulatory and policy changes in the medical marijuana industry, the market data available is limited and unreliable. Federal, and state laws prevent widespread participation and hinder market research. Therefore, market research and projections by the Company of estimated total retail sales, demographics, demand, and similar consumer research, are based on assumptions from limited and unreliable market data, and generally represent the personal opinions of our management team as of the date of this document.
We do not have long-term agreements or guaranteed price or delivery arrangements with most of our suppliers. The loss of a significant supplier would require us to rely more heavily on our other existing suppliers or to develop relationships with new suppliers. Such a loss may have an adverse effect on our product offerings and our business.
Consistent with industry practice, we do not have guaranteed price or delivery arrangements with most of our suppliers. We generally make our purchases through purchase orders, although we have some internal processing capabilities through City Trees. As a result, we have experienced and may in the future experience inventory shortages or price increases on certain products. Furthermore, our industry occasionally experiences significant product supply shortages, and we sometimes experience customer order backlogs due to the inability of certain suppliers to make available to us certain products as needed. We cannot assure you that suppliers will maintain an adequate inventory of products to fulfill our orders on a timely basis, or at all, or that we will be able to obtain particular products on favorable terms, or at all. Additionally, we cannot assure you that product lines currently offered by suppliers will continue to be available to us. A decline in the supply or continued availability of the products of our suppliers, or a significant increase in the price of those products, could reduce our sales and negatively affect our operating results.
In addition, some of our suppliers have the ability to terminate their relationships with us at any time, or to decide to sell, or increase their sales of, their products through other channels. Although we believe there are numerous suppliers with the capacity to supply the products we distribute, the loss of one or more of our major suppliers could have an adverse effect on our product offerings and our business. Such a loss would require us to rely more heavily on our other existing suppliers, develop relationships with new suppliers or undertake our own manufacturing, which may cause us to pay higher prices for products. Any termination, interruption or adverse modification of our relationship with a key supplier or a significant number of other suppliers would likely adversely affect our operating income, cash flow and future prospects.
We are subject to certain operating risks for which our insurance coverage may not be adequate.
Our operations are subject to hazards inherent in the medical marijuana industry, such as equipment defects, malfunction and failures, natural disasters which result in fires, accidents and explosions that can cause personal injury, loss of life, suspension of operations, damage to facilities, business interruption and damage to or destruction of property, equipment and the environment, labor disputes, and changes in the regulatory environment. These risks could expose the Company to substantial liability for personal injury, wrongful death, property damage, pollution, and other environmental damages. The frequency and severity of such incidents will affect operating costs, insurability and relationships with customers, employees and regulators.
We continuously monitor our operations for quality control and safety. However, there are no assurances that our safety procedures will always prevent such damages. Although we maintain insurance coverage that we believe to be adequate and customary in the industry, there can be no assurance that such insurance will be adequate to cover its liabilities. In addition, there can be no assurance that we will be able to maintain adequate insurance in the future at rates we consider reasonable and commercially justifiable. The occurrence of a significant uninsured claim, a claim in excess of the insurance coverage limits maintained by the Company, or a claim at a time when it is not able to obtain liability insurance, could have a material adverse effect on us, our ability to conduct normal business operations and on our business, financial condition, results of operations and cash flows in the future.
We may have uninsured or uninsurable risk.
We may be subject to liability for risks against which we cannot insure or against which we may elect not to insure due to the high cost of insurance premiums or other factors. The payment of any such liabilities would reduce the funds available for our normal business activities. Payment of liabilities for which the Company does not carry insurance may have a material adverse effect on our financial position and operations.
We may issue debt.
From time to time, the Company may enter into transactions to acquire assets or the shares of other organizations. These transactions may be financed in whole or in part with debt, which may increase our debt levels above industry standards for companies of similar size. Depending on future exploration and development plans, the Company may require additional equity and/or debt financing that may not be available or, if available, may not be available on favorable terms to us. Neither our articles nor our by-laws limit the amount of indebtedness that the Company may incur. As a result, the level of our indebtedness from time to time, could impair its ability to obtain additional financing on a timely basis to take advantage of business opportunities that may arise.
Certain remedies shareholders may seek against our officers and directors may be limited and such officers and directors may be entitled to indemnification by the Company.
Our governing documents provide that the liability of our Board and officers is eliminated to the fullest extent allowed under the laws of the State of Nevada. Thus, the Company and the shareholders of the Company may be prevented from recovering damages for alleged errors or omissions made by the members of the Board and its officers. Our governing documents also provide that the Company will, to the fullest extent permitted by law, indemnify members of the Board and its officers for certain liabilities incurred by them by virtue of their acts on behalf of the Company.
We are dependent on attracting new customers.
Our success depends on our ability to attract and retain customers. There are many factors which could impact our ability to attract and retain clients, including but not limited to our ability to continually produce desirable and effective products, the successful implementation of our client-acquisition plan and continued growth in the aggregate number of patients selecting medical marijuana as a treatment option. Our failure to acquire and retain patients as customers would have a material adverse effect on our business, operating results and financial condition.
We are subject to interest rate risks.
Interest rate risk is the risk that future cash flows will fluctuate as a result of changes in market interest rates. Our debt and borrowings are all at fixed interest rates, therefore the interest rate risk is limited to potential changes on cash held with financial institutions. As interest on these balances is negligible, the Company considers interest rate risk to be immaterial.
We are subject to certain credit risks.
We are exposed to credit risk through our cash and cash equivalents. Credit risk arises from deposits with banks and outstanding receivables. We do not hold any collateral as security but mitigate this risk by dealing only with what management believes to be financially sound counterparties and, accordingly, do not anticipate significant loss for non-performance.
Risks Related to the IGH Merger Agreement
The fact that IGH is a private company limits our access to some information that may be relevant to the transaction. This may result in a transaction that is not as profitable as we suspect.
By definition, very little public information exists about private companies, which required us to make decisions on whether to pursue the transactions contemplated by the IGH Merger Agreement on the basis of limited information provided by the respective parties, which may result in one or both of the business combinations being less profitable than we suspected, if at all.
We may not be able to realize the anticipated benefits from the transactions contemplated by the IGH Merger Agreement.
The successful completion of the transactions contemplated by the IGH Merger Agreement may not yield the anticipated benefits or the benefits may not occur in the anticipated time frame. Moreover, the ability to realize the benefits in the expected time frame may be materially adversely affected by a number of factors. Each of the proposed acquisitions to date has placed, and future acquisitions could continue to place, significant demands on both our and IGH’s administrative, operational and financial resources and may also result in the assumption of unexpected liabilities and may divert management’s attention from the operation of the Company’s and IGH’s business.
Risks related to the Ownership of our Common Stock
Our directors and officers control a large portion of our Common Stock.
The officers and directors of the Company currently own approximately 26.12% of the issued and outstanding shares of Common Stock. Our shareholders nominate and elect the Board, which generally has the ability to control the acquisition or disposition of our assets, and the future issuance of our Common Stock or other securities. Accordingly, for any matters with respect to which a majority vote of our Common Stock may be required by law, our directors and officers may have the ability to control such matters. Because the directors and officers control a substantial portion of such Common Stock, investors may find it difficult or impossible to replace our directors if they disagree with the way our business is being operated.
Because our common stock is deemed a low-priced “Penny” stock, an investment in our common stock should be considered high risk and subject to marketability restrictions.
Since our common stock is a penny stock, as defined in Rule 3a51-1 under the Exchange Act, it will be more difficult for investors to liquidate their investment. The SEC defines “penny stock” to be any equity security that has a market price (as defined) less than $5.00 per share or an exercise price of less than $5.00 per share, subject to certain exceptions. The shares of Common Stock are covered by the penny stock rules pursuant to Rule 15g-9 under the Exchange Act, which impose additional sales practice requirements on broker-dealers who sell to persons other than established customers and “accredited investors”. The term “accredited investor” refers generally to institutions with assets in excess of $5,000,000 or individuals with a net worth in excess of $1,000,000 or annual income exceeding $200,000 or $300,000 jointly with their spouse. The penny stock rules require a broker-dealer, prior to a transaction in a penny stock not otherwise exempt from the rules, to deliver a standardized risk disclosure document in a form prepared by the SEC which provides information about penny stocks and the nature and level of risks in the penny stock market. The broker-dealer also must provide the customer with current bid and offer quotations for the penny stock, the compensation of the broker-dealer and its salesperson in the transaction and monthly account statements showing the market value of each penny stock held in the customer’s account. The bid and offer quotations, and the broker-dealer and salesperson compensation information, must be given to the customer orally or in writing prior to effecting the transaction and must be given to the customer in writing before or with the customer’s confirmation. In addition, the penny stock rules require that prior to a transaction in a penny stock not otherwise exempt from these rules, the broker-dealer must make a special written determination that the penny stock is a suitable investment for the purchaser and receive the purchaser’s written agreement to the transaction. These disclosure requirements may have the effect of reducing the level of trading activity in the secondary market for the securities of the Company that are captured by the penny stock rules. Consequently, the penny stock rules may affect the ability of broker-dealers to trade our securities. Management believes that the penny stock rules could discourage investor interest in and limit the marketability of our Common Stock.
Financial Industry Regulatory Authority sales practice requirements may also limit a stockholder’s ability to buy and sell our common stock, which could depress the price of our common stock.
In addition to the “penny stock” rules described above, the U.S. Financial Industry Regulatory Authority (“FINRA”) has adopted rules that require a broker-dealer to have reasonable grounds for believing that an investment is suitable for a customer before recommending an investment to a customer. Prior to recommending speculative, low priced securities to non-institutional customers, broker-dealers must make reasonable efforts to obtain information about the customer’s financial status, tax status, investment objectives, and other information. Pursuant to the interpretation of these rules, FINRA believes that there is a high probability that speculative, low priced securities will not be suitable for at least some customers. Thus, the FINRA requirements make it more difficult for broker-dealers to recommend our Common Stock to customers which may limit an investor’s ability to buy and sell our Common Stock, have an adverse effect on the market for our Common Stock, and thereby negatively impact the price of our Common Stock.
Our Common Stock is subject to liquidity risks.
In the United States, our Common Stock trades on the OTCQB. The OTCQB is an inter-dealer, over-the-counter market that provides significantly less liquidity than other national or regional exchanges. Securities traded on the OTCQB are usually thinly traded, highly volatile, have fewer market makers and are not followed by analysts. The SEC’s order handling rules, which apply to NASDAQ-listed securities, do not apply to securities quoted on the OTCQB. Quotes for stocks listed on the OTCQB are not listed in newspapers. Therefore, prices for securities traded solely on the OTCQB may be difficult to obtain and holders of our securities may be unable to resell their securities at or near their original acquisition price, or at any price.
We cannot predict at what prices our Common Stock will trade and there can be no assurance that an active trading market will develop or be sustained. Commencing in January 2019, our Common Stock began trading on the CSE. Because our Common Stock has traded for a very short period of time on the CSE, we have not developed any meaningful liquidity on this exchange and we cannot guaranty that we will do so in the future. There is a significant liquidity risk associated with an investment in the Company.
The shares of our Common Stock we may issue in the future and the options we may issue in the future may have an adverse effect on the market price of our Common Stock and cause dilution to investors.
We may issue shares of Common Stock and warrants to purchase Common Stock pursuant to private offerings and we may issue options to purchase Common Stock to our executive officers pursuant to their employment agreements. The sale, or even the possibility of sale, of shares pursuant to a separate offering or to executive officers could have an adverse effect on the market price of our Common Stock or on our ability to obtain future financing.
Our amended and restated articles of incorporation and bylaws could discourage acquisition proposals, delay a change in control or prevent other transactions.
Provisions of our amended and restated articles of incorporation and bylaws, as well as provisions of Nevada Corporation Law, may discourage, delay or prevent a change in control of the Company or other transactions that you as a shareholder may consider favorable and may be in your best interest. The amended and restated articles of incorporation and bylaws contain provisions that: authorize the issuance of shares of “blank check” preferred stock that could be issued by our board of directors (“Board of Directors”) to increase the number of outstanding shares and discourage a takeover attempt; limit who may call special meetings of shareholders; and require advance notice for business to be conducted at shareholder meetings, among other anti-takeover provisions
Our directors have the authority to issue common and preferred shares without shareholder approval, and preferred shares can be issued with such rights, preferences, and limitations as may be determined by our board of directors. The rights of the holders of Common Stock will be subject to, and may be adversely affected by, the rights of any holders of preferred stock that may be issued in the future. Although we authorized a series A preferred stock in 2017, we presently have no commitments or contracts to issue any shares of preferred stock. Authorized and unissued preferred stock could delay, discourage, hinder or preclude an unsolicited acquisition of our company, could make it less likely that shareholders receive a premium for their shares as a result of any such attempt, and could adversely affect the market prices of and the voting and other rights, of the holders of outstanding shares of our Common Stock.
We do not expect to pay any cash dividends for the foreseeable future.
The continued operation and expansion of our business may require substantial funding. Accordingly, we do not anticipate that we will pay any cash dividends on shares of our Common Stock for the foreseeable future. Any determination to pay dividends in the future will be at the discretion of our Board of Directors and will depend upon results of operations, financial condition, contractual restrictions, including any indebtedness we may incur, restrictions imposed by applicable law and other factors our Board of Directors deems relevant.
Our stock price may be volatile and you may not be able to sell your shares for more than what you paid.
Our stock price may be subject to significant volatility, and you may not be able to sell shares of Common Stock at or above the price you paid for them. The trading price of our Common Stock has been subject to fluctuations in the past and the market price of our Common Stock could continue to fluctuate in the future in response to various factors, including, but not limited to: quarterly variations in operating results; our ability to control costs and improve cash flow; announcements of innovations or new products by us or by our competitors; changes in investor perceptions; and new products or product enhancements by us or our competitors.
If securities analysts or industry analysts downgrade our shares, publish negative research or reports, or cease to publish reports about our business, our share price and trading volume could decline.
The trading market for our Common Stock is influenced by the research and reports that industry or securities analysts publish about us, our business and our industry. If one or more analysts adversely change their recommendation regarding our shares or our competitors’ stock, our share price would likely decline. If one or more analysts cease coverage of us or fail to regularly publish reports on us, we could lose visibility in the financial markets, which in turn could cause our share price or trading volume to decline. As a result, the market price for our Common Stock may decline below the offering price and you might not be able to resell your shares of our Common Stock at or above the offering price.
Our principal offices are located at 11767 South Dixie Highway, Suite 115, Miami, Florida 33156. We currently maintain an administrative office at 3355 SW 59th Avenue, Miami, Florida 33155. Alternative Solutions and the Oasis LLCs lease space for a dispensary and administrative offices at 1800 Industrial Road, Suite 180, Las Vegas, Nevada 89102, and for a cultivation and processing facility at 203 E. Mayflower Avenue, North Las Vegas, Nevada 89030.
On July 6, 2014, Alternative Solutions entered into a Lease Agreement with 18000 Industrial, LLC (the “1800 Industrial Lease”) for the lease of a 1,000 square foot storefront and 5,900 square foot warehouse for the use of a medical marijuana dispensary and related uses, as approved by the City of Las Vegas and State of Nevada. Pursuant to the terms of the 1800 Industrial Lease, basic monthly rent payments are currently $8,195.45 per month and will increase to $8,441.30 per month beginning January 1, 2019. The 1800 Industrial Lease has an initial term of five (5) years with one renewal option to renew for a five year term with rent starting at the then market rate for like spaces, but not less than rent for the fifth year of the original lease term. On June 13, 2018, Alternative Solutions assigned the 1800 Industrial Lease to the Company.
On December 3, 2016, Serenity Wellness Growers, LLC (“Serenity Wellness”) and SFC Leasing, LP (“SFC”), entered into a Standard Industrial/Commercial Single Tenant Lease with an Option to Purchase Lease Rider (the “SFC Lease”) pursuant to which Serenity Wellness leases approximately 22,000 square feet from SFC used for the cultivation, processing, and other legal uses related to medical marijuana, including general office/administrative, storage, sales and distribution. The SFC Lease has an initial term of five years and two months with one renewal option for a five year term. Pursuant to the terms of the SFC Lease, Serenity Wellness paid an initial security deposit in the amount of $50,000. On January 12, 2016, the parties entered into a First Amendment to the SFC Lease to modify the base rent schedule and to require two additional $50,000 security deposits for a total security deposit equal to $150,000. Any unused portion of the $150,000 security deposit shall be applied to the purchase price in the event that Serenity Wellness exercises the Option to Purchase. Pursuant to the terms of that certain Second Amendment to the SFC Lease between the parties, monthly base rent is currently $21,500 per month and will increase to $25,000 per month for the period from January 1, 2019 through December 31, 2019, and $29,000 per month for the period from January 1, 2020 through February 28, 2021. As required under the SFC Lease, on June 11, 2018 Serenity Wellness and SFC entered into a Landlord Consent agreeing to the change in control in the ownership of Serenity Wellness following the Oasis Acquisition.
From time to time, we may become involved in various lawsuits and legal proceedings, which arise, in the ordinary course of business. However, litigation is subject to inherent uncertainties, and an adverse result in these or other matters may arise from time to time that may harm our business. We are currently not aware of any such legal proceedings or claims that we believe will have a material adverse effect on our business, financial condition or operating results.
Item 4. Mine Safety Disclosures.
Not Applicable.
PART II
Item 5. Market for Registrant’s Common Equity, Related Stockholder Matters and Issuer Purchases of Equity Securities.
The Company was initially incorporated on March 31, 2011 as Adelt Design, Inc. Effective August 21, 2013; our common stock became eligible for quotation on the OTC Bulletin Board under the symbol ADSN. On November 12, 2014, CLS Labs acquired 6,250,000 shares, or 55.6%, of the outstanding common stock of the Company from its founder, Larry Adelt. As a condition to CLS Labs’ purchase of these shares, and pursuant to five stock purchase agreements each dated November 12, 2014, five people or entities unaffiliated with the Company purchased an aggregate of 4,984,376 shares of common stock in the Company from twenty-four stockholders other than Mr. Adelt. The total number of shares acquired by these five purchasers represented 44.3% of the Company’s outstanding shares of common stock. On November 20, 2014, we adopted amended and restated articles of incorporation therein changing the Company’s name to CLS Holdings USA, Inc. Effective December 10, 2014 we changed our stock symbol to “CLSH” to reflect the name change of the Company. Our common stock is currently eligible for quotation on the OTC Markets’ OTCQB under the symbol “CLSH”. Commencing in January 2019, we also listed our Common Stock on the CSE under the symbol “CLSH”. We have no outstanding shares of preferred stock.
The following table sets forth the range of high and low sales prices on the OTCQB for the applicable periods on a post-Reverse-Split basis.
|
Common Stock |
||||||||
|
High ($) |
Low ($) |
|||||||
|
Fiscal Year Ended May 31, 2019: |
||||||||
|
Fourth Quarter |
$ | 0.4737 | $ | 0.24 | ||||
|
Third Quarter |
$ | 0.95 | $ | 0.26 | ||||
|
Second Quarter |
$ | 1.20 | $ | 0.80 | ||||
|
First Quarter |
$ | 1.26 | $ | 0.82 | ||||
|
Fiscal Year Ended May 31, 2018: |
||||||||
|
Fourth Quarter |
$ | 0.75 | $ | 0.55 | ||||
|
Third Quarter |
$ | 0.94 | $ | 0.41 | ||||
|
Second Quarter |
$ | 0.50 | $ | 0.27 | ||||
|
First Quarter |
$ | 0.57 | $ | 0.07 | ||||
At August 19, 2019, we had 126,420,345 outstanding shares of Common Stock and approximately 70 shareholders of record. The number of record holders was determined from the records of our transfer agent and does not include beneficial owners of common stock whose shares are held in the names of bank, brokers and other nominees. We have no outstanding shares of preferred stock.
Dividend Policy
We have not paid any cash dividends on our Common Stock to date. Any future decisions regarding dividends will be made by our Board of Directors. We do not anticipate paying dividends in the foreseeable future, but expect to retain earnings to finance the growth of our business. Our Board of Directors has complete discretion on whether to pay dividends. Even if our Board of Directors decides to pay dividends, the form, frequency and amount will depend upon our future operations and earnings, capital requirements and surplus, general financial condition, contractual restrictions and other factors that the Board of Directors may deem relevant.
Purchases of Equity Securities by the Small Business Issuer and Affiliates
None.
Securities Authorized for Issuance under Equity Compensation Plans
The following table summarizes as of May 31, 2019, the shares of our common stock subject to outstanding awards or available for future awards under our equity compensation plans.
|
Plan Category |
|
Number of shares to be issued upon exercise of outstanding options, warrants and rights |
|
|
Weighted-average exercise price of outstanding options, warrants and rights |
|
|
Number of shares remaining available for future issuance under equity compensation plans (excluding shares reflected in the first column) |
|
|||
|
Equity compensation plans approved by security holders |
|
|
-- |
|
|
|
-- |
|
|
|
-- |
|
|
Equity compensation plans not approved by security holders (1) |
|
|
-- |
|
|
|
-- |
|
|
|
-- |
|
|
Total |
|
|
-- |
|
|
|
-- |
|
|
|
-- |
|
|
(1) |
Pursuant to their respective employment agreements, Jeffrey Binder is entitled to receive annual stock options, exercisable at the fair market value of our common stock on the date of grant, in an amount equal to 2% of our annual EBITDA up to $42.5 million and 4% of our annual EBITDA in excess of $42.5 million; and Andrew Glashow is entitled to receive annual restricted stock grants in an amount equal to 1% of our annual EBITDA. We are currently unable to determine the number of shares that could be granted under these plans. |
Penny Stock Regulations
The SEC has adopted regulations which generally define “penny stock” to be an equity security that has a market price of less than $5.00 per share. Our Common Stock, when and if a trading market develops, may fall within the definition of penny stock and be subject to rules that impose additional sales practice requirements on broker-dealers who sell such securities to persons other than established customers and accredited investors (generally those with assets in excess of $1,000,000, or annual incomes exceeding $200,000 individually, or $300,000, together with their spouse).
For transactions covered by these rules, the broker-dealer must make a special suitability determination for the purchase of such securities and have received the purchaser’s prior written consent to the transaction. Additionally, for any transaction, other than exempt transactions, involving a penny stock, the rules require the delivery, prior to the transaction, of a risk disclosure document mandated by the SEC relating to the penny stock market. The broker-dealer also must disclose the commissions payable to both the broker-dealer and the registered representative, current quotations for the securities and, if the broker-dealer is the sole market-maker, the broker-dealer must disclose this fact and the broker-dealer’s presumed control over the market. Finally, monthly statements must be sent disclosing recent price information for the penny stock held in the account and information on the limited market in penny stocks. Consequently, the penny stock rules may restrict the ability of broker-dealers to sell our Common Stock and may affect the ability of investors to sell their Common Stock in the secondary market.
Item 6. Selected Financial Data.
Not applicable.
Item 7. Management’s Discussion and Analysis of Financial Condition and Results of Operations.
History and Outlook
We were incorporated on March 31, 2011 as Adelt Design, Inc. to manufacture and market carpet binding art. Production and marketing of carpet binding art never commenced. On November 20, 2014, we adopted amended and restated articles of incorporation, thereby changing our name to CLS Holdings USA, Inc. Effective December 10, 2014, we effected a reverse stock split of our issued and outstanding common stock at a ratio of 1-for-0.625 (the “Reverse Split”), wherein 0.625 shares of our Common Stock were issued in exchange for each share of Common Stock issued and outstanding.
On April 29, 2015, the Company, CLS Labs and the Merger Sub consummated the Merger, whereby the Merger Sub merged with and into CLS Labs, with CLS Labs remaining as the surviving entity. As a result of the Merger, we acquired the business of CLS Labs and abandoned our previous business. As such, only the financial statements of CLS Labs are included herein.
CLS Labs was originally incorporated in the state of Nevada on May 1, 2014 under the name RJF Labs, Inc. before changing its name to CLS Labs, Inc. on October 24, 2014. It was formed to commercialize a proprietary method of extracting cannabinoids from cannabis plants and converting the resulting cannabinoid extracts into concentrates such as oils, waxes, edibles and shatter. These concentrates may be ingested in a number of ways, including through vaporization via electronic cigarettes (“e-cigarettes”), and used for a variety of pharmaceutical and other purposes. Testing in conjunction with two Colorado growers of this extraction method and conversion process has revealed that it produces a cleaner, higher quality product and a significantly higher yield than the cannabinoid extraction processes currently existing in the marketplace.
On April 17, 2015, CLS Labs took its first step toward commercializing its proprietary methods and processes by entering into the Colorado Arrangement through its wholly owned subsidiary, CLS Labs Colorado, with certain Colorado entities, including PRH. During 2017, we suspended our plans to proceed with the Colorado Arrangement due to regulatory delays and have not yet determined if or when we will pursue them again.
We have been issued a U.S. patent with respect to our proprietary method of extracting cannabinoids from cannabis plants and converting the resulting cannabinoid extracts into concentrates such as oils, waxes, edibles and shatter. These concentrates may be ingested in a number of ways, including through vaporization via electronic cigarettes, and used for a variety of pharmaceutical and other purposes. Internal testing of this extraction method and conversion process has revealed that it produces a cleaner, higher quality product and a significantly higher yield than the cannabinoid extraction processes currently existing in the marketplace. We have not yet commercialized our proprietary process. We plan to generate revenues through licensing, fee-for-service and joint venture arrangements related to our proprietary method of extracting cannabinoids from cannabis plants and converting the resulting cannabinoid extracts into saleable concentrates.
We intend to monetize our extraction and conversion method and generate revenues through (i) the licensing of our patented proprietary methods and processes to others, (ii) the processing of cannabis for others, and (iii) the purchase of cannabis and the processing and sale of cannabis-related products. We plan to accomplish this through the acquisition of companies, the creation of joint ventures, through licensing agreements, and through fee-for-service arrangements with growers and dispensaries of cannabis products. We believe that we can establish a position as one of the premier cannabinoid extraction and processing companies in the industry. Assuming we do so, we then intend to explore the creation of our own brand of concentrates for consumer use, which we would sell wholesale to cannabis dispensaries. We believe that we can create a “gold standard” national brand by standardizing the testing, compliance and labeling of our products in an industry currently comprised of small, local businesses with erratic and unreliable product quality, testing practices and labeling. We also plan to offer consulting services through Cannabis Life Sciences Consulting, LLC, which will generate revenue by providing consulting services to cannabis-related businesses, including growers, dispensaries and laboratories, and driving business to our processing facilities.
On December 4, 2017, we entered into the Acquisition Agreement with Alternative Solutions to acquire the outstanding equity interests in the Oasis LLCs. Pursuant to the Acquisition Agreement, as amended, we paid a non-refundable deposit of $250,000 upon signing, which was followed by an additional payment of $1,800,000 on February 5, 2018, for an initial 10% of Alternative Solutions and each of the subsidiaries. At the closing of our purchase of the remaining 90% of the ownership interests in Alternative Solutions and the Oasis LLCs, which occurred on June 27, 2018, we paid the following consideration: $5,995,543 in cash, a $4.0 million promissory note due in December 2019, and $6,000,000 in shares of our Common Stock. The cash payment of $5,995,543 was less than the $6,200,000 payment originally contemplated because we assumed an additional $204,457 of liabilities. The Oasis Note is secured by all of the membership interests in Alternative Solutions and the Oasis LLCs and by the assets of the Oasis LLCs. We received final regulatory approval to own the membership interests in the Oasis LLCs on December 12, 2018.
On October 31, 2018, the Company, CLS Massachusetts, Inc., a Massachusetts corporation and a wholly-owned subsidiary of the Company (“CLS Massachusetts”), and In Good Health, Inc., a Massachusetts corporation (“IGH”), entered into an Option Agreement (the “IGH Option Agreement”). Under the terms of the IGH Option Agreement, CLS Massachusetts has an exclusive option to acquire all of the outstanding capital stock of IGH (the “IGH Option”) during the period beginning on the earlier of the date that is one year after the effective date of the conversion and December 1, 2019 and ending on the date that is 60 days after such date. If CLS Massachusetts exercises the IGH Option, the Company, a wholly-owned subsidiary of the Company and IGH will enter into a merger agreement (the form of which has been agreed to by the parties) (the “IGH Merger Agreement”). At the effective time of the merger contemplated by the IGH Merger Agreement, CLS Massachusetts will pay a purchase price of $47,500,000, subject to reduction as provided in the IGH Merger Agreement, payable as follows: $35 million in cash, $7.5 million in the form of a five-year promissory note, and $5 million in the form of restricted Common Stock of the Company, plus $2.5 million as consideration for a non-competition agreement with IGH’s President, payable in the form of a five-year promissory note. IGH and certain IGH stockholders holding sufficient aggregate voting power to approve the transactions contemplated by the IGH Merger Agreement have entered into agreements pursuant to which such stockholders have, among other things, agreed to vote in favor of such transactions. On October 31, 2018, as consideration for the IGH Option, we made a loan to IGH, in the principal amount of $5,000,000, subject to the terms and conditions set forth in that certain loan agreement, dated as of October 31, 2018 between IGH as the borrower and the Company as the lender. The loan is evidenced by a secured promissory note of IGH, which bears interest at the rate of 6% per annum and matures on October 31, 2021. To secure the obligations of IGH to us under the loan agreement and the promissory note, the Company and IGH entered into a security agreement dated as of October 31, 2018, pursuant to which IGH granted to us a first priority lien on and security interest in all personal property of IGH. If we do not exercise the Option on or prior to the date that is 30 days following the end of the option period, the loan amount will be reduced to $2,500,000 as a break-up fee, subject to certain exceptions set forth in the IGH Option Agreement. On August 26, 2019, the parties amended the IGH Option Agreement to, among other things, delay the closing until January 2020.
On September 13, 2018, we entered into a non-binding letter of intent (the “CannAssist LOI”) with CannAssist, LLC (“CannAssist”) setting forth the terms and conditions upon which we propose to acquire an 80% ownership interest in CannAssist. CannAssist plans to build out a recreationally licensed cultivation grow facility in Leicester, Massachusetts.
On January 29, 2019, we made a line of credit loan to CannAssist, in the principal amount of up to $500,000, subject to the terms and conditions set forth in that certain Loan Agreement, dated as of January 29, 2019 between CannAssist as the Borrower and the Company as the Lender (the “CannAssist Loan Agreement”). The Loan is evidenced by a secured promissory note of CannAssist (the “CannAssist Note”), which bears interest at the rate of 8% per annum and is personally guaranteed by the two equity owners of CannAssist. CannAssist has drawn down $325,000 on the CannAssist Note. At August 26, 2019, CannAssist had drawn down $325,000 on the CannAssist Note.
To secure the obligations of CannAssist to the Company under the CannAssist Loan Agreement and the CannAssist Note, the Company and CannAssist entered into a Security Agreement dated as of January 29, 2019, pursuant to which CannAssist granted to the Company a first priority lien on and security interest in all personal property of CannAssist.
On March 11, 2019, the Company, through our wholly-owned subsidiary, CLS Massachusetts, entered into a membership interest purchase agreement (the “CannAssist Purchase Agreement”) with CannAssist, each of the members of CannAssist, and David Noble, as the members’ representative. Mr. Noble currently serves as the President of IGH, an entity that we hold an option to acquire. After conducting diligence regarding the cost of the planned buildout of the CannAssist facility, the parties jointly decided to terminate the CannAssist Purchase Agreement effective August 26, 2019. The CannAssist Note will be due and payable in full not later than February 28, 2020.
On January 4, 2018, the Attorney General of the United States issued new written guidance concerning the enforcement of federal laws relating to marijuana. The Attorney General’s memorandum stated that previous DOJ guidance specific to marijuana enforcement, including the memorandum issued by former Deputy Attorney General James Cole on August 29, 2013 (as amended on February 14, 2014, the “Cole Memo”) is unnecessary and is rescinded, effective immediately. The Cole Memo told federal prosecutors that in states that had legalized marijuana, they should use their prosecutorial discretion to focus not on businesses that comply with state regulations, but on illicit enterprises that create harms like selling drugs to children, operating with criminal gangs, and selling across state lines. Although the rescission did not change federal law, as the Cole Memo and other DOJ guidance documents were not themselves laws, the rescission removed the DOJ’s formal policy that state-regulated cannabis businesses in compliance with the Cole Memo guidelines should not be a prosecutorial priority. Notably, former Attorney General Sessions’ rescission of the Cole Memo has not affected the status of the FinCen memorandum issued by the Department of Treasury, which remains in effect. This memorandum outlines Bank Secrecy Act-compliant pathways for financial institutions to service state-sanctioned cannabis business, which echoed the enforcement priorities outlined in the Cole Memo. In addition to his rescission of the Cole Memo, former Attorney General Sessions issued a one-page memorandum known as the “Sessions Memorandum.” The Sessions Memorandum explains the DOJ’s rationale for rescinding all past DPJ cannabis enforcement guidance, claiming that Obama-era enforcement policies are “unnecessary” due to existing general enforcement guidance adopted in the 1980s. Although the Sessions Memorandum emphasizes that cannabis is a federally illegal Schedule I controlled substance, it does not otherwise instruct U.S. Attorneys to consider the prosecution of cannabis-related offenses a DOJ priority, and in practice, most U.S. Attorneys have not changed their prosecutorial approach to date. However, due to the lack of specific direction in the Sessions Memorandum as to the priority federal prosecutors should ascribe to such cannabis activities, there can be no assurance that the federal government will not seek to prosecute cases involving cannabis businesses that are otherwise compliant with state law. See “Risk Factors.”
We incurred a net loss of $27,619,057 for the year ended May 31, 2019, resulting in an accumulated deficit as of May 31, 2019 of $46,188,151. These conditions raise substantial doubt about our ability to continue as a going concern.
Results of Operations for the Years Ended May 31, 2019 and May 31, 2018
Revenues
We had revenue of $8,459,048 and $0 during the years ended May 31, 2019 and 2018, respectively. The increase in revenue for the year ended May 31, 2019 was due to our acquisition of the Oasis LLCs. Our cannabis dispensary accounted for $5,492,312 of our revenue, and our cannabis production accounted for $2,966,736 of our revenue.
Cost of Goods Sold
Our cost of goods sold for the year ended May 31, 2019 was $4,836,166, compared to cost of goods sold of $0 for the year ended May 31, 2018. The increase in cost of goods sold for the year ended May 31, 2019 was due to our acquisition of the Oasis LLCs. Cost of goods sold primarily consisted of $4,502,759 of product cost, $68,546 of licensing fees, $140,779 of sales tax, $50,422 of other costs of goods sold, $31,905 of supplies and materials, and $41,755 of freight. Our gross margin for the year ended May 31, 2019 was 43%.
Selling, General and Administrative Expenses
Selling, general and administrative expenses, or SG&A, increased by $23,356,017, or approximately 750%, to $26,472,057 during the year ended May 31, 2019, compared to $3,116,040 for the year ended May 31, 2018. The increase in SG&A expenses for the year ended May 31, 2019 was primarily due to one-time financing and acquisition costs attributable to the acquisition of the Oasis LLCs, as well as ongoing operational costs of the Oasis LLCs, which we acquired during the year ended May 31, 2019.
SG&A for the year ended May 31, 2019 included one-time cash and non-cash financing and acquisition costs in the aggregate amount of $15,857,068 compared to $503,655 during the year ended May 31, 2018. The major components of these costs were as follows: the fair value of additional warrants and special warrants issued due to the failure to meet certain registration statement filing requirements in connection with the Westpark offering and the Canaccord offering in the amount of $8,084,522; the fair value of the special warrants and compensation broker warrants issued to Canaccord in connection with our sale of the special warrants in the amount of $4,340,465; broker and agent fees and commissions in the amount of $2,350,993; the fair value of 700,000 shares of common stock issued to Star Associates, which is affiliated with one of our directors, for services in connection with the Oasis transaction of $490,000; a foreign exchange loss on conversion of the Canaccord funds from Canadian to U.S. dollars in the amount of $403,588; and a redemption premium on the YA PN II Note in the amount of $187,500.
The increase in SG&A expense during fiscal 2019 was also attributable to an aggregate of $4,611,948 in costs associated with operating the Oasis LLCs, which we acquired effective June 27, 2018. As a result, we did not incur any of these costs during fiscal 2018, but we also had revenue associated with these operations during fiscal 2019, as described above, that we did not have during fiscal 2018. The major components of the operating costs associated with the Oasis LLCs are as follows: payroll and related costs of $2,401,167; lease, facilities and office costs of $838,051; sales, marketing, and advertising of $453,968; depreciation and amortization of $287,954; insurance of $240,451; taxes and licenses of $204,329; professional fees of $141,326; and travel costs of $44,702.
Finally, SG&A also increased by an aggregate of $3,390,656 during fiscal 2019 as a result of the ongoing implementation of other aspects of our business plan and increases in our general corporate overhead to an aggregate of $6,003,041 during fiscal 2019 from $2,612,385 during the prior year. The major components of these increases compared to fiscal 2018 are as follows: investor relations costs increased by $1,117,423; legal fees increased by $777,137; consulting fees increased by $409,654; non-cash compensation increased by $288,503; facilities, office, and general costs increased by $306,032; accounting fees increased by $271,439; and travel costs increased by $220,468. The increase in these expenses was primarily due to our increased efforts to identify and close on equity and debt financings during fiscal 2019.
Interest Expense, Net
Our interest expense, net of interest income, was $4,447,993 for the year ended May 31, 2019, a decrease of $261,947, or 6%, compared to $4,709,940 for the year ended May 31, 2018. The decrease in interest expense was primarily due to a decrease in the value of derivative liabilities in excess of the principal amount of notes payable by $1,870,337 compared to the prior year. The decline in interest expense for fiscal 2019 was partially offset by an increase in the amortization of discounts on convertible notes payable to third parties of $1,042,058 compared to the prior year; and an increase in interest accrued on third party debt by $997,038 compared to the prior fiscal year. We also had interest income of $174,247 from the IGH Note Receivable and $4,011 from the CannAssist Note Receivable for total interest income of $178,258 during the year ended May 31, 2019 from notes receivable, compared to $0 during the prior fiscal year.
Overall, the decrease in interest expense for the year ended May 31, 2019 compared to the prior fiscal year was primarily due to the decrease in the interest associated with the derivative financial liabilities recorded in connection with the convertible notes and warrants that we issued during the year ended May 31, 2018. The derivative liabilities associated with these convertible notes, in the aggregate original principal amount of $1,688,000, together with, in some cases, our issuance of warrants, accounted for $1,882,996 of interest expense during the year ended May 31, 2018, compared to $12,659 during the 2019 fiscal year, which decrease occurred because these convertible notes were converted during fiscal 2019. This decrease was partially offset by an increase in accrued interest during the 2019 fiscal year in the amount of $997,038 due to an increase in the principal amount of outstanding debt. At May 31, 2019, we had outstanding debt due to third parties in the amount of $22,360,230, compared to $1,690,000 at May 31, 2018.
Gain on Settlement of Debt
During the year ended May 31, 2018, we recognized a gain on the settlement of accounts payable in the amount of $3,480 because we repaid an account using our Common Stock. There was no comparable transaction during the current year.
Loss on Revaluation of Contingent Liability
During the year ended May 31, 2019, we revalued the performance-based contingent liability which may be due to the sellers in connection with the Oasis Acquisition. Pursuant to our evaluation, we increased the amount of the contingent liability from $678,111 to $1,000,000 to reflect the increase in sales generated by the Oasis LLCs during the year ended May 31, 2019, which increases the likelihood that the performance criteria will be met and that the payment will be due. This resulted in a charge to operations in the amount of $321,889 during the year ended May 31, 2019. There was no such charge during fiscal 2018.
Loss on Modification of Debt
During the year ended May 31, 2019, we recognized a loss on modification of debt in the amount of $0 compared to $29,145 during the year ended May 31, 2018. The prior year loss was related to the amendment of the Old Main 8% Note (defined below).
Loss on Note Exchange
During the year ended May 31, 2019, we recognized a loss on the exchange of debt in the amount of $0 compared to $404,082 during the prior year. This loss during fiscal 2018 was related to the exchange of the April 2015 Note for our Common Stock.
Loss on Extinguishment of Debt
During the year ended May 31, 2019, we recognized a loss on the extinguishment of debt in the amount of $0 compared to $989,032 during fiscal 2018. The fiscal 2018 loss was related to the exchange of the Old Main 8% Note for our Common Stock.
Prepayment Penalty
During the year ended May 31, 2019, we incurred a prepayment penalty in the amount of $0 compared to $137,000 during the prior year. This penalty was related to the redemption of the FirstFire note payable during fiscal 2018.
Change in Fair Value of Derivative Liability
During the years ended May 31, 2019 and 2018, we had outstanding convertible promissory notes that contained conversion price reset features, which required us to value and record a derivative liability related to this provision on a quarterly basis during the year ended May 31, 2018. This revaluation resulted in a charge to operations in the amount of $195,725 for the year ended May 31, 2018. On June 1, 2019, we adopted ASU 2017-11, and reclassified the derivative liability in the amount of $1,265,751 to additional paid-in capital and are no longer required to revalue the derivative liability on a quarterly basis. We had no gain or loss in connection with derivative liabilities during the year ended May 31, 2019. Management uses a lattice model to estimate the fair value of derivative liabilities.
Net Loss
For the reasons stated above, our net loss for the year ended May 31, 2019 was $27,619,057 compared to $9,577,484 for the year ended May 31, 2018, an increase of $18,041,573 or 188%. The net loss per diluted share for the year ended May 31, 2019 was $0.27, compared to a net loss per diluted share of $0.24 for the year ended May 31, 2018. These amounts were computed based on the weighted average of 102,869,612 and 39,224,613 shares outstanding during the fiscal years ended May 31, 2019 and 2018, respectively.
Liquidity and Capital Resources
The following table summarizes our total current assets, liabilities and working capital at May 31, 2019 and 2018:
|
May 31, |
May 31, |
|||||||
|
2019 |
2018 |
|||||||
|
Current Assets |
$ | 12,677,566 | $ | 54,374 | ||||
|
Current Liabilities |
$ | 6,924,543 | $ | 2,689,148 | ||||
|
Working Capital (Deficit) |
$ | 5,753,023 | $ | (2,634,774 |
) |
|||
At May 31, 2019, we had working capital of $5,753,023, an increase of $8,387,797 from the working capital deficit of $2,634,774 we had at May 31, 2018. Our working capital at May 31, 2019, includes $10,525,791 of cash. The increase in working capital was a result of our successful financing activities during the year ended May 31, 2019, including $15,535,978 in proceeds from the sale of equity and $18,369,000 from the issuance of convertible notes. We are presently expanding the Oasis and City Trees businesses in Nevada and are considering other potential acquisitions, including our previously announced potential acquisition of IGH in Massachusetts. Our working capital needs will likely continue to increase, and if we require additional funds to meet them, we will seek additional debt or equity financing. We have operated at a loss since inception.
Cash flows used in operating activities was $10,164,542 during the year ended May 31, 2019, an increase of $8,739,244, or 613%, compared to $1,425,298 during the year ended May 31, 2018. In deriving cash flows used in operating activities from the net loss for fiscal 2019, there were a large number or non-cash items that were added back to the net loss for fiscal 2019. The significant items were as follows: a $8,084,522 financing penalty associated with our issuance of warrants and special warrants, and an additional $4,744,053 related to the value of warrants and units issued to placement agents and non-cash offering costs. During fiscal 2018, comparable addbacks were only $503,655. In addition, during fiscal 2019, we issued shares of our common stock to officers, consultants and in a settlement, the aggregate value of which was $991,824. We did not make any such issuances during fiscal 2018. We also incurred $3,576,161 of amortization of debt discounts associated with our convertible debt during fiscal 2019 compared to $2,534,103 during fiscal 2018. During fiscal 2018, we had excess derivatives associated with our warrants and convertible debt in the amount of $1,940,439, stock-based compensation issued to management and consultants in the amount of $794,607 and a loss on extinguishment of debt associated with the amendment of the Old Main notes payable in the amount of $989,032, which collectively were types of addbacks to our net loss that we did not incur during fiscal 2019 and which partially offset the increase in net cash used in operating activities when compared to the respective net losses for fiscal 2019 versus fiscal 2018.
Finally, our cash used in operating activities was affected by changes in the components of working capital. These changes occurred primarily due to our acquisition and operation of the Oasis LLCs during fiscal 2019; we did not own the Oasis LLCs during fiscal 2018. The overall net change in the components of working capital resulted in an increase in cash used in operating activities in the amount of $565,751 during the year ended May 31, 2019, compared to a decrease in the amount of $625,480 during the prior fiscal year. The more significant changes were as follows: we used $340,880 in cash to increase inventory levels due to increased sales; we used $484,609 to pay down accounts payable and accrued expenses, compared to an increase in accounts payable and accrued expenses of $300,421 in the prior year, which was unrelated to the Oasis LLCs; and $120,417 to pay accrued compensation, compared to an increase accrued compensation of $216,667 in the prior fiscal year. These changes from the prior fiscal year were a result of the availability of cash from our financing efforts during fiscal 2019. We also used $292,769 in cash for deposits and prepaid licensing fees in connection with the Oasis LLCs, compared to $0 in the prior year. We also increased our cash used in operating activities to adjust for the non-cash increase in accounts receivable of $128,134 and interest receivable of $178,258, compared, in both cases, to $0 in the prior fiscal year. Lastly, our increase in cash used in operating activities was partially offset by an increase in accrued interest of $995,941, compared to $11,476 in the prior year. This related to interest accrued on the convertible debentures issued during fiscal 2019.
Cash used in investing activities were $12,169,972 for the year ended May 31, 2019, an increase of $10,119,972, or 494%, compared to $2,050,000 during the year ended May 31, 2018. During the year ended May 31, 2019, we made cash payments in the amount of $5,982,710, net of cash received of $14,612, for our investment in Alternative Solutions, and made loans to In Good Health and CannAssist in the aggregate amount of $5,150,000. We also made cash payments for property and equipment in the amount of $1,037,262 related to our Expansion Plan.
Cash flows provided by financing activities were $32,807,341 for the year ended May 31, 2019, an increase of $29,357,389 or 851%, compared to $3,449,952 during the year ended May 31, 2018. During the year ended May 31, 2019, we received cash in the amount of $18,369,000 from the issuance of convertible debentures in the 2018 U.S. Convertible Debenture Offering and 2018 Convertible Debenture Offering, and $15,535,978 from the sale of equity to Navy Capital and in the Canaccord Special Warrant Offering. During the year ended May 31, 2018, we only received $1,655,000 from the sale of convertible debentures and $1,460,917 from the sale of equity. During the year ended May 31, 2019, cash flows from financing activities were reduced by principal payments in the amount of $1,060,000 on notes payable, principal payments of $37,500 on convertible notes payable, and a principal payment of $137 on a related party note payable.
Third Party Debt
The table below summarizes the status of our third party debt and reflects whether such debt remains outstanding, has been repaid, or has been converted into or exchanged for our Common Stock:
|
Name of Note |
Original Principal Amount |
Outstanding or Repaid |
Payment Details |
||||
|
April 2015 Note |
$ | 200,000 |
Repaid |
Repaid in part and exchanged in part |
|||
|
Old Main 8% Note |
$ | 200,000 |
Repaid |
Exchanged for 4,500,000 shares |
|||
|
FirstFire Note |
$ | 363,000 |
Repaid |
Repaid |
|||
|
Darling Capital Note |
$ | 550,000 |
Repaid |
Converted into 1,808,000 shares |
|||
|
Efrat Investments Note |
$ | 55,000 |
Repaid |
Converted into 183,040 shares |
|||
|
Todd Blatt |
$ | 210,000 |
Repaid |
Repaid |
|||
|
AJG Group |
$ | 100,000 |
Repaid |
Repaid |
|||
|
YA II PN Note |
$ | 750,000 |
Repaid |
Repaid |
|||
| $ | 250,000 |
Repaid |
$250,000 plus interest converted into 700,616 shares of Common Stock. |
||||
| $ | 250,000 |
Repaid |
$250,000 plus interest converted into 640,068 shares of Common Stock. |
||||
|
Oasis Note |
$ | 4,000,000 |
Outstanding |
Due December 2019; repaid in part as a result of settlement with 4Front |
|||
|
2018 U.S. Convertible Debentures |
$ | 5,857,000 |
Outstanding |
Due October 26-31, 2021 |
|||
|
2018 Convertible Debentures |
$ | 12,012,000 |
Outstanding |
Due December 2021 |
|||
April 2015 Note
On April 29, 2015, we issued a convertible promissory note (the “April 2015 Note”) to an unaffiliated individual in the amount of $200,000. Interest accrued on the April 2015 Note at a rate of 15% per annum. On the first anniversary of the April 2015 Note, all then-accrued interest was due thereunder. Thereafter, principal together with accrued interest was due in eight (8) equal quarterly payments, in arrears, commencing on July 1, 2016. All outstanding principal and any accumulated unpaid interest thereon was due and payable on the third anniversary of note. At the holder's election, at any time prior to payment or prepayment of the April 2015 Note in full, all principal and accrued interest under the April 2015 Note could be converted in whole, but not in part, into our securities. For each dollar converted, the holder would receive two shares of Common Stock and a three-year warrant to purchase 1.33 shares of Common Stock at $0.75 per share. During the year ended May 31, 2017, we repaid principal in the amount of $100,000 and interest in the amount of $53,837 on this note.
On September 20, 2017, we entered into an exchange agreement, whereby we agreed to exchange the April 2015 Note for 1,500,000 shares of our Common Stock. The holder of the April 2015 Note had previously sold it for $105,219, which represented the balance due by us, to StarForce Media, Inc., an entity that is not affiliated with us. We recognized a loss on this exchange in the amount of $404,082, which was charged to operations during the twelve months ended May 31, 2018. We also expensed the remaining discount in the amount of $18,155 to interest expense during the twelve months ended May 31, 2018.
Old Main 8% Note and Equity Line
On March 18, 2016, we issued Old Main Capital, LLC (“Old Main”) an 8% Convertible Promissory Note (the “Old Main 8% Note”) in the principal amount of $200,000 for Old Main’s commitment to enter into an equity line transaction with us and prepare all of the related transaction documents. The Old Main 8% Note bore interest at the rate of 8% per annum. On October 6, 2016, we amended the Old Main 8% Note, among other documents (the “First Amendment”) to defer the commencement of amortization payments on the Old Main 8% Note so that they commenced at the earlier of February 3, 2017 or on the date the registration statement with respect to the underlying shares had been declared effective by the SEC. On such date, we were required to begin to redeem 1/6th of the face amount of the Old Main 8% Note and any accrued but unpaid interest on a monthly basis. Such amortization payment could be made, at our option, in cash or, subject to certain conditions, in our Common Stock pursuant to a conversion rate equal to the lower of (a) $1.07 or (b) 75% of the lowest VWAP in the twenty (20) consecutive trading days ending on the trading day that is immediately prior to the applicable conversion date.
On November 28, 2016, we entered into a Second Amendment to the Old Main 8% Note issued on March 18 (the “Second Amendment”) to amend the Old Main 8% Note, among other documents, as amended by the First Amendment, in certain respects. Pursuant to the Second Amendment, among other things, the Old Main 8% Note was converted from an installment note to a “balloon” note, with all principal and interest on the Old Main 8% Note due on March 18, 2017; the Fixed Conversion Price associated with the Old Main 8% Note was changed to a variable conversion price equal to the lesser of the prior Fixed Conversion Price or 75% of the lowest VWAP in the fifteen trading days ending on the trading day immediately prior to the conversion date; our ability to repay the Old Main 8% Note with our Common Stock was deleted except pursuant to a voluntary conversion by Old Main; and Old Main was prohibited from selling, per trading day, an amount of our Common Stock in excess of the greater of $5,000 or 25% of the average number of shares of Common Stock sold per day for the five trading days preceding the day of sale multiplied by the average daily VWAP during the immediately preceding 5-trading day period.
On March 27, 2017, we entered into the third amendment to the Old Main 8% Note, which, among other things, increased the outstanding amount due under the Old Main 8% Note as of March 18, 2017 by 5%. In exchange for doing so, Old Main agreed to extend the maturity of the Old Main 8% Note until July 1, 2017 and to suspend conversions under the Old Main 8% Note until July 1, 2017.
On July 6, 2017, we entered into the fourth amendment to the Old Main 8% Note (the “Fourth Amendment”) to further amend the terms of the Old Main 8% Note. Pursuant to the Fourth Amendment, the maturity date of the Old Main 8% Note was extended to July 15, 2017 and the outstanding balance of the Old Main 8% Note as of June 30, 2017 was increased by multiplying it by 1.075. The Fourth Amendment was effective on June 30, 2017.
On August 23, 2017, we entered into the fifth amendment to the Old Main 8% Note (the “Fifth Amendment”) to further amend the terms of the Old Main 8% Note. Pursuant to the Fifth Amendment, the maturity date of the Old Main 8% Note was extended to September 15, 2017 and the outstanding balance remained unchanged. The Fifth Amendment was effective on July 15, 2017.
On September 25, 2017, but effective as of September 15, 2017, we entered into an exchange agreement, whereby we agreed to exchange the Old Main 8% Note for 4,500,000 shares of our Common Stock. Pursuant to an oral agreement with the original holder of the Old Main 8% Note, principal due under the Old Main 8% note was increased by $96,862 to a total of $322,612 prior to the date on which the exchange of the 8% Note for Common Stock occurred.
On April 18, 2016, we also entered into an equity line agreement with Old Main whereby we may issue and sell to Old Main, at our option from time to time, up to $4,000,000 of our Common Stock at a purchase price equal to 80% of the lowest VWAP of our Common Stock during a five day “Valuation Period.”
On October 6, 2016, we entered into an amendment to the equity line Agreement to amend the new commitment period, which is 24 months from the date of this amendment. Second, the equity line agreement was amended to prohibit us from delivering a subsequent put notice from the beginning of any “Valuation Period” until the fourth trading day immediately following the closing associated with the prior put notice. Third, the beneficial ownership limitation was amended to increase the beneficial ownership limitation to 9.99% and to remove the ability of Old Main to increase or decrease the beneficial ownership limitation. We have not “put” any Common Stock to Old Main under the equity line Agreement.
FirstFire Note
On November 15, 2017, we entered into a securities purchase agreement with FirstFire Global Opportunities Fund, LLC (“FirstFire”), whereby FirstFire agreed to purchase a 5% senior convertible promissory note in the aggregate principal amount of $363,000 (the “FirstFire Note”) from us due, subject to the terms therein, seven (7) months from the date of issuance, for a purchase price of $330,000.
The FirstFire Note bore interest at the rate of 5% per annum. Any past due accrued and unpaid interest to be paid under the FirstFire Note was to bear interest at the lesser of 15% per annum or the maximum rate permitted by applicable law. At any time prior to the 180th day following the date of issuance, we could prepay all or any portion of the principal amount of the FirstFire Note and any accrued and unpaid interest by paying the following amounts: (i) within the initial 90 days after the date of issuance: 115% multiplied by the principal amount then due plus accrued interest; and (ii) from the 91st day through the 180th day after the date of issuance: 125% multiplied by the principal amount then due plus accrued interest.
The FirstFire Note was convertible at any time into shares of our Common Stock, at the option of the holder, at an initial conversion rate equal $0.40 per share of Common Stock (the “Fixed Conversion Price”). Any time on or after the 180th day after the issuance of the FirstFire Note, the conversion price would equal the lower of (a) the Fixed Conversion Price or (b) 75% of the lowest traded price of our Common Stock in the 20 consecutive trading days immediately prior to the day that we receive the applicable conversion notice.
On the closing date, we also issued FirstFire a three-year Common Stock purchase warrant to purchase 350,000 shares of our Common Stock at an initial exercise price of $0.75 per share and agreed to issue FirstFire promptly following the closing date 250,000 shares of our restricted Common Stock as a commitment fee to enter into the purchase agreement and prepare all of the related transaction documents.
During the three months ended February 28, 2018, an event occurred that triggered the reduction of the FirstFire Fixed Conversion Price from $0.40 per share to $0.3125 per share.
On May 9, 2018, we entered into an amendment to the FirstFire Note, whereby we agreed to make a $50,000 payment on or before May 14, 2018 and a $450,000 payment on or before May 31, 2018 to repay the FirstFire Note in full. We also agreed to issue an additional warrant to purchase 25,000 shares of our Common Stock. In exchange, the note holder agreed that it would not convert the FirstFire Note until after May 31, 2018. During the twelve months ended May 31, 2018, we made payments of $500,000 on this note, which amounts repaid the FirstFire Note in full.
Darling Capital Note
On February 5, 2018, we entered into a securities purchase agreement with Darling, whereby Darling agreed to purchase an 8% convertible promissory note in the aggregate principal amount of $550,000 (the “Darling Note”) from us due, subject to the terms therein, eighteen (18) months from the date of issuance, for a purchase price of $500,000.
Darling could, at its option, convert all or a portion of the Darling Note and accrued but unpaid interest into shares of Common Stock at a conversion price of $0.3125 per share. On the closing date, we also issued Darling a three-year Common Stock purchase warrant to purchase 400,000 shares of our Common Stock at an initial exercise price of $0.75 per share.
On June 12, 2018, we received a conversion notice from Darling notifying us that it had converted $550,000 in principal and $15,000 of accrued interest into 1,808,000 shares of our Common Stock.
Efrat Investments Note
On February 16, 2018, we entered into a securities purchase agreement with Efrat, whereby Efrat agreed to purchase an 8% convertible promissory note in the aggregate principal amount of $55,000 (the “Efrat Note”) from us due, subject to the terms therein, eighteen (18) months from the date of issuance, for a purchase price of $50,000.
Efrat could, at its option, convert all or a portion of the Efrat Note and accrued but unpaid interest into shares of Common Stock at a conversion price of $0.3125 per share. On the closing date, we also issued Efrat a three-year Common Stock purchase warrant to purchase 40,000 shares of our Common Stock at an initial exercise price of $0.75 per share.
On August 9, 2018, we received a conversion notice from Efrat notifying us that it had converted $55,000 in principal and $2,200 of accrued interest into 183,040 shares of our Common Stock.
The YA II PN, Ltd. Notes
On May 11, 2018, we entered into a securities purchase agreement with YA II, pursuant to which we agreed to sell to YA II, in two closings, (i) convertible debentures in the aggregate principal amount of $1,250,000, plus accrued interest, which may be converted into shares of our Common Stock, at the discretion of either YA II or us in accordance with the terms of the debentures, and (ii) five-year warrants to purchase an aggregate of 3,125,000 shares of our Common Stock at $0.60 per share of Common Stock. At the first closing, which occurred on May 14, 2018, we issued a $750,000 debenture to YA II and warrants to purchase 1,875,000 shares of our Common Stock. At the second closing, which occurred on July 20, 2018, we issued a $500,000 debenture to YA II and warrants to purchase 1,250,000 additional shares of our Common Stock.
The debentures bear interest at the rate of 8% per annum. If an event of default occurs and for so long as such event of default remains uncured, the interest rate on the debentures shall immediately become 15% per annum and shall remain at such increased interest rate until the applicable event of default is cured.
Commencing on December 1, 2018 and on the first day of each month thereafter through July 1, 2019, subject to certain exceptions, we shall pay to YA II one-eighth of the principal amount of the debentures, plus accrued and outstanding interest (the “Installment Amount”), plus 20% of the of the Installment Amount for Installment Amounts due within 180 days following the date of execution of the purchase agreement, and 25% of the Installment Amount for Installment Amounts due thereafter in cash or by converting such Installment Amount into shares of our Common Stock. if we have met the applicable conditions for such a conversion and as long as the conversion does not exceed certain maximum amounts. Each Installment Amount will be deferred to the maturity date if the daily dollar volume-weighted average price of our Common Stock equals or exceeds $0.40 per share for each of the 10 consecutive days preceding the fifth trading day prior to the respective installment date.
Pursuant to the terms of the debentures, YA II may elect to convert any portion of the principal and accrued interest under the debentures into our Common Stock at a fixed conversion price of $0.40 per share. The fixed conversion price may change if certain dilutive events or issuances occur. In addition, we may, at our sole discretion, make an Installment Payment using our Common Stock if certain conditions have been met. In such case, the applicable conversion price would be equal to 75% of the VWAP of our Common Stock during the fifteen consecutive trading days immediately preceding such conversion. During the three months ended August 31, 2018, a reset event occurred. As a result, the conversion price of the first YA II PN Note, in the principal amount of $750,000, was reduced to $0.34 per share of Common Stock.
During the year ended May 31, 2019, YA II converted a total of $280,247, which consisted of $250,000 of principal and $30,247 of accrued interest, into 700,616 shares of Common Stock. On January 8, 2019, YA II converted $256,027, of which $250,000 was principal and $6,027 was accrued interest, into 640,068 shares of Common Stock.
On February 28, 2019, we redeemed all of the convertible debentures issued to YA II in full for total cash consideration of $964,787.
Blatt Note
On February 7, 2018, we issued a note payable to Todd Blatt in the amount of $210,000. This note accrued interest at a rate of 6% per annum and was due on February 7, 2019. This note along with $5,627 of accrued interest was paid on July 20, 2018.
AJG Group Note
On February 7, 2018, we issued a note payable to AJG Group in the amount of $200,000. This note accrued interest at a rate of 6% per annum and was due on February 7, 2019. We made a principal payment in the amount of $100,000 on this note on March 30, 2018; we then made an additional principal payment of $100,000, together with accrued interest in the amount of $3,337, on July 9, 2018.
Oasis Note
On June 27, 2018, we closed on the purchase of the remaining 90% of the membership interests of Alternative Solutions and the Oasis LLCs. The closing occurred pursuant to the Acquisition Agreement dated December 4, 2017, as amended. On such date, we made the payments to indirectly acquire the remaining 90% of the Oasis LLCs, which were equal to cash in the amount of $5,995,543, a $4.0 million promissory note due in December 2019 (the “Oasis Note”), and 22,058,823 shares of our Common Stock. The cash payment of $5,995,543 was less than the $6,200,000 payment originally contemplated because we assumed an additional $204,457 in liabilities. The Oasis Note bears interest at the rate of 6% per annum. The principal amount of the Oasis Note was reduced in August 2019, in accordance with the terms of the Acquisition Agreement, as a result of the settlement of the dispute between the former owners of Alternative Solutions and 4Front Advisors, a consultant to Alternative Solutions. The terms of the settlement with 4Front Advisors are confidential. The balance of the Oasis Note may be prepaid at any time without penalty. The Oasis Note is secured by all of the membership interests in Alternative Solutions and the Oasis LLCs and by the assets of the Oasis LLCs.
2018 U.S. Convertible Debenture Offering
Between October 25, 2018 and November 2, 2018, we entered into six subscription agreements, pursuant to which we agreed to sell, for an aggregate purchase price of $5,857,000, $5,857,000 in original principal amount of convertible debentures in minimum denominations of $1,000 each. The debentures bear interest, payable quarterly, at a rate of 8% per annum, with interest during the first eighteen (18) months following their issuance, being payable by increasing the then-outstanding principal amount of the debentures. The debentures mature on a date that is three years following their issuance. The debentures are convertible into units at a conversion price of $0.80 per unit. Each unit consists of (i) one share of our Common Stock, par value $.001 and (ii) one-half of one warrant, with each warrant exercisable for three years to purchase a share of Common Stock at a price of $1.10. The debentures have other features, such as mandatory conversion in the event our Common Stock trades at a particular price over a specified period of time and required redemption in the event of a “Change in Control” of the Company. The debentures are unsecured obligations of the Company and rank pari passu in right of payment of principal and interest with all other unsecured obligations of the Company. Navy Capital and its affiliates purchased $5,000,000 in principal amount of debentures, with the remaining $857,000 in principal amount being purchased by several unaffiliated purchasers. The debentures include a provision for the capitalization of accrued interest on a quarterly basis; at May 31, 2019, accrued interest in the aggregate amount of $199,259 has been capitalized, and the aggregate principal amount of the debentures is $6,056,259.
If the debentures are converted, the warrants that would be issued are exercisable from time to time, in whole or in part for three years. The warrants have anti-dilution provisions that provide for an adjustment to the exercise price in the event of a future sale of our Common Stock at a lower price, subject to certain exceptions as set forth in the warrant. The warrants also provide that we can force their exercise at any time after the bid price of our Common Stock exceeds $2.20 for a period of 20 consecutive business days.
On July 26, 2019, we entered into amendments to these convertible debentures with four of the purchasers, pursuant to which we agreed to adjust the conversion price of the original debentures if, in general, we issue or sell common stock, or warrants or options exercisable for common stock, or any other securities convertible into common stock, in a capital raising transaction, at a consideration per share, or exercise or conversion price per share, as applicable, less than the conversion price of the original debentures in effect immediately prior to such issuance (a “Dilutive Issuance”). In such case, the conversion price of the original debentures will be reduced to such issuance price (the “Adjusted Conversion Price”). The amendments also provides that, if a Dilutive Issuance occurs, the warrant to be issued upon conversion will be exercisable at a price equal to 137.5% of the Adjusted Conversion Price at the time of conversion of the debenture (the “Revised Warrant Exercise Price”). If a Dilutive Issuance occurs, the form of warrant attached to the subscription agreement shall be amended to change the Initial Exercise Price, as defined therein, to be the Revised Warrant Exercise Price.
2018 Convertible Debenture Offering
On December 12, 2018, we entered into an agency agreement with two Canadian agents regarding a private offering of up to $40 million of convertible debentures of the Company at an issue price of $1,000 per debenture. The agents sold the convertible debentures on a commercially reasonable efforts private placement basis. Each debenture is convertible into units of the Company at the option of the holder at a conversion price of $0.80 per unit at any time prior to the close of business on the last business day immediately preceding the maturity date of the debentures, being the date that is three (3) years from the closing date of the offering (the “2018 Convertible Debenture Offering”). Each unit will be comprised of one share of Common Stock and a warrant to purchase one-half of a share of Common Stock. Each warrant will be exercisable for one share of Common Stock at a price of $1.10 per warrant for a period of 36 months from the closing date.
We closed the 2018 Convertible Debenture Offering on December 12, 2018, issuing $12,012,000 million in 8% senior unsecured convertible debentures at the initial closing. At the closing, we paid the agents: (A)(i) a cash fee of $354,000 for advisory services provided to us in connection with the offering; (ii) a cash commission of $720,720, equivalent to 6.0% of the aggregate gross proceeds received at the closing of the offering; (B)(i) an aggregate of 184,375 units for advisory services; and (ii) a corporate finance fee equal to 375,375 units, which is the number of units equal to 2.5% of the aggregate gross proceeds received at the closing of the offering divided by the conversion price; and (C)(i) an aggregate of 442,500 advisory warrants; and (ii) 900,900 broker warrants, which was equal to 6.0% of the gross proceeds received at the closing of the offering divided by the conversion price. The debentures include a provision for the capitalization of accrued interest on a quarterly basis; at May 31, 2019, accrued interest in the amount of $291,971 has been capitalized, and the principal amount of the debenture is $1,303,971.
The debentures are unsecured obligations of the Company, rank pari passu in right of payment of principal and interest and were issued pursuant to the terms of a debenture indenture, dated December 12, 2018, between the Company and Odyssey Trust Company as the debenture trustee. The debentures bear interest at a rate of 8% per annum from the closing date, payable on the last business day of each calendar quarter. For a period of 18 months from the closing date, any interest payable shall automatically accrue and be capitalized to the principal amount of the debentures and shall thereafter be deemed to be part of the principal amount of the convertible debentures.
Beginning on the date that is four (4) months plus one (1) day following the closing date, we may force the conversion of all of the principal amount of the then outstanding debentures at the conversion price on not less than 30 days’ notice should the daily volume weighted average trading price of our Common Stock be greater than $1.20 per share for the preceding 10 consecutive trading days.
Upon a change of control of the Company, holders of the debentures have the right to require us to repurchase their debentures at a price equal to 105% of the principal amount of the debentures then outstanding plus accrued and unpaid interest thereon. The debentures also contain standard anti-dilution provisions.
If, at the time of exercise of any warrant in accordance with the warrant indenture, there is no effective registration statement under the U.S. Securities Act of 1933, as amended (the “U.S. Securities Act”) covering the resale by the holder of a portion of the shares of Common Stock to be issued upon exercise of the warrant, or the prospectus contained therein is not available for the resale of the shares of Common Stock by the holder under the U.S. Securities Act by reason of a blackout or suspension of use thereof, then the warrants may be exercised, in part for that portion of the shares of Common Stock not registered for resale by the holder under an effective registration statement or in whole in the case of the prospectus not being available for the resale of such shares of Common Stock, at such time by means of a “cashless exercise” in which the holder shall be entitled to receive a number of shares of Common Stock equal to the quotient obtained by dividing [(A-B) (X)] by (A), where: A = the last volume weighted average price (“VWAP”) for the trading day immediately preceding the time of delivery of the exercise form giving rise to the applicable “cashless exercise”; B = the exercise price of the warrant; and X = the number of shares of Common Stock that would be issuable upon exercise of the warrant in accordance with the terms of such warrant if such exercise were by means of a cash exercise rather than a cashless exercise.
Pursuant to the agency agreement, we granted the agents an option to increase the offering by an additional $6 million in principal amount of debentures, which option was not exercised by the agents prior to the closing date of the offering.
Pursuant to the agency agreement and the subscription agreements signed by investors in the offering, we granted certain registration rights to the holders of the debentures pursuant to which we agreed to prepare and file a registration statement with the SEC to register the resale by the original purchasers of the debentures of the shares of Common Stock issuable upon conversion of the debentures or exercise of the warrants.
We intend to use the net proceeds of the 2018 Convertible Debenture Offering to complete Phase I of the Expansion Plan and/or for general working capital purposes.
Related Party Debt
David Lamadrid Note
On February 26, 2018, we entered into a securities purchase agreement with Mr. Lamadrid, our former President and Chief Financial Officer, whereby Mr. Lamadrid agreed to purchase an 8% convertible promissory note in the aggregate principal amount of $31,250 (the “Lamadrid Note”) from us due, subject to the terms therein, eighteen (18) months from the date of issuance.
Mr. Lamadrid could, at his option, convert all or a portion of the Lamadrid Note and accrued but unpaid interest into shares of Common Stock at a conversion price of $0.3125 per share. On the closing date, we also issued Mr. Lamadrid a three-year Common Stock purchase warrant to purchase 25,000 shares of our Common Stock at an initial exercise price of $0.75 per share.
On August 21, 2018, we received a conversion notice from Mr. Lamadrid notifying us that he had converted $31,250 in principal and $1,247 of accrued interest into 103,989 shares of our Common Stock.
Koretsky and Affiliate Notes
Between August 11, 2015 and May 31, 2017, we borrowed an aggregate of $1,657,000 from Frank Koretsky, a director of the Company, and $150,000 from CLS CO 2016, LLC and $465,000 from Newcan, two entities that are affiliated with Mr. Koretsky. These loans were unsecured, accrued interest between 6% and 15% per year, were due either on demand or within three years after the date of the applicable note, and, in some cases, were convertible into shares of our Common Stock and warrants at rates between $0.25 and 1.07 per share. Effective on May 31, 2017, we entered into the Omnibus Loan Amendment Agreement, whereby the portion of these loans that was advanced prior to December 31, 2017 was converted into our Common Stock, together with accrued interest on these loans. As a result of these conversions, Mr. Koretsky, CLS CO 2016, LLC and Newcan converted an aggregate of $1,485,000, $150,000, and $460,000 in principal, and $130,069, 49,247 and $7,747 in accrued interest, into an aggregate of 6,460,276, 636,988 and 1,870,988 shares of Common Stock at $0.25 per share. Pursuant to the Omnibus Loan Amendment Agreement, the conversion rate on all of the loans made by Mr. Koretsky, CLS CO 2016, LLC and Newcan was reduced, if applicable, to $0.25 per share and Mr. Koretsky and his affiliates gave up the right to receive warrants upon conversion. Thus, each of Mr. Koretsky, CLS CO 2016, LLC and Newcan received 4,560,849, 488,159 and 1,433,841 shares of Common Stock in excess of what they would have received had they converted their loans into Common Stock prior to the effective date of the Omnibus Loan Amendment Agreement.
Between June 1, 2017 and May 31, 2018, we borrowed an aggregate of $145,000 from Newcan Investment Partners, LLC, an entity that is affiliated with Mr. Koretsky. These loans were unsecured, accrued interest at 10% per year, were due either on demand or within three years after the date of the applicable note, and were convertible into shares of our Common Stock and warrants at $0.25 per share. On January 10, 2018, effective December 1, 2017, we entered into the Second Omnibus Loan Agreement with Newcan and Mr. Binder. The Second Omnibus Loan Agreement provides that the conversion price of all outstanding convertible promissory notes issued to Newcan as of the date of the agreement would be increased from $0.25 to $0.3125 per share of Common Stock. The remaining terms of such notes remain unchanged. Following the Second Omnibus Loan Agreement, on March 12, 2018, Newcan converted all of its outstanding convertible loans, which totaled $956,658 in principal and $98,098 in accrued interest, into a total of 3,375,220 shares of our Common Stock.
On August 6, 2018, we issued a convertible promissory note to Newcan, an entity owned by Frank Koretsky, a director of the Company, in the amount of $75,000 (the “Newcan Convertible Note 8”), to finalize the terms of repayment with respect to a certain loan made to the Company by Newcan on May 4, 2018, which was converted into 196,336 shares of Common Stock on October 23, 2018.
Binder Notes
Between June 1, 2015 and May 31, 2017, we borrowed an aggregate of $251,800 from Jeffrey Binder, a director and officer of the Company. These loans were unsecured, accrued interest between 6% and 10% per year, were due either on demand or within three years after the date of the applicable note, and, in some cases, were convertible into shares of our Common Stock and warrants at rates between $.25 and 1.07 per share. Effective on May 31, 2017, we entered into the Omnibus Loan Amendment Agreement, whereby the portion of these loans that was advanced prior to May 31, 2017 was converted into our Common Stock, together with accrued interest on these loans. As a result of these conversions, Mr. Binder converted an aggregate of $442,750 in principal and $19,427 in accrued interest, into an aggregate of 1,848,708 shares of Common Stock at $.25 per share. Pursuant to the Omnibus Loan Amendment Agreement, the conversion rate on all of the loans made by Mr. Binder was reduced, if applicable, to $.25 per share and Mr. Binder gave up the right to receive warrants upon conversion. Thus, Mr. Binder received 1,127,061 shares of Common Stock in excess of what he would have received had he converted his loans into Common Stock prior to the effective date of the Omnibus Loan Amendment Agreement.
Between June 1, 2017 and March 31, 2018, we borrowed an aggregate of $204,881 from Mr. Binder. These loans were unsecured, accrued interest at 10% per year, were due either on demand or within three years after the date of the applicable note, and were convertible into shares of our Common Stock and warrants at $0.25 per share. On January 10, 2018, effective December 1, 2017, we entered into the Second Omnibus Loan Agreement with Newcan and Mr. Binder. The Second Omnibus Loan Agreement provides that the conversion price of all outstanding convertible promissory notes issued to Mr. Binder as of the date of the agreement would be increased from $0.25 to $0.3125 per share of Common Stock. The remaining terms of such notes remain unchanged. Following the Second Omnibus Loan Agreement, on March 12, 2018, Mr. Binder converted all of his outstanding convertible loans, which totaled $464,698 in principal and $43,058 in accrued interest, into a total of 1,624,819 shares of our Common Stock.
On April 6, 2018, we issued Binder Convertible Note 9, in the amount of $37,500, to Mr. Binder with respect to certain compensation payable to Mr. Binder as of February 28, 2018, which was repaid in full on August 7, 2018.
Omnibus Loan Amendment Agreements
On May 31, 2017, we entered into an Omnibus Loan Amendment Agreement (the “Omnibus Loan Amendment”) with Jeffrey Binder, Frank Koretsky, Newcan Investment Partners LLC and CLS CO 2016, LLC (collectively, the “Insiders”). Pursuant to the Omnibus Loan Amendment, we agreed with the Insiders to amend certain terms of loans the Insiders made to us for working capital purposes, which loans were initially demand loans, and, except for certain loans made in 2017, were later memorialized as convertible loans (the “Insider Loans”), in exchange for the agreement of the Insiders to convert all Insider Loans where funds were advanced prior to January 1, 2017, which total $2,537,750, plus $166,490 of accrued interest thereon, into an aggregate of 10,816,960 shares of our Common Stock, and forego the issuance of warrants to purchase our Common Stock upon conversion. This resulted in the issuance of an additional 7,609,910 shares compared to the original number of shares issuable upon conversion of the Insider Loans prior to the Omnibus Loan Agreement. We valued the shares at $0.125, which was the market price of our stock at the conversion date, and charged the amount of $951,239 to loss on modification of debt during the twelve months ended May 31, 2017.
We entered into the Omnibus Loan Amendment in order to ease the debt burden on us and prevent us from defaulting on the Insider Loans. Pursuant to the Omnibus Loan Amendment, the following amendments were made to the Insider Loans: (a) we reduced the conversion price on the Insider Loans from between $0.75 and $1.07 per share of Common Stock to $0.25 per share of Common Stock, in those cases where the conversion price was greater than $0.25, which reduced conversion price exceeds the closing price of the Common Stock during the last three months; (b) we deleted the requirement to issue warrants to purchase our Common Stock upon conversion of the Insider Loans; (c) we amended one Insider Loan to permit conversion of only the portion of the Insider Loan related to services that were provided to us prior to January 1, 2017; and (d) we amended the terms of the Insider Loans where funds were advanced on or after January 1, 2017, which Insider Loans were not converted into our Common Stock, to provide for, where not already the case, a 10% interest rate per annum, a $0.25 conversion price per share of Common Stock, and the deletion of the requirement that we issue warrants to purchase our Common Stock upon conversion of such Insider Loans.
On January 10, 2018, effective December 1, 2017, we entered into the Second Omnibus Loan Agreement with Jeffrey I. Binder, an officer and director of the Company, and Newcan, an entity owned by Frank Koretsky, a director of the Company. The Second Omnibus Loan Agreement provides that the conversion price of all outstanding convertible promissory notes issued to either Mr. Binder or Newcan as of the date of such agreement would be increased from $0.25 to $0.3125 per share of Common Stock. The remaining terms of such notes remain unchanged.
Sales of Equity
WestPark Offering
During February and March 2018, we held four closings of the WestPark Offering, whereby we sold units for $1.25 per unit. Each unit consisted of four shares of Common Stock and one warrant to purchase one share of our Common Stock for $0.75 per share. We sold a total of 1,368,250 units in the WestPark Offering for aggregate gross proceeds of $1,710,313, and aggregate net proceeds of $1,460,918 after deduction of placement agent commissions, a non-accountable expense allowance and expenses associated with the offering. We valued the warrants associated with the WestPark Offering using the Black-Scholes valuation model, and allocated gross proceeds in the amount of $1,370,764 to the common stock and $339,549 to the warrants. We also issued WestPark Capital, Inc., as placement agent, a five-year warrant to purchase 205,238 units at a price of $1.25 per unit. The unit warrant were valued at $503,655, which amount was charged to operations during the twelve months ended May 31, 2018. We used the proceeds of the WestPark Offering towards deposits due in connection with our acquisition of Oasis and for general corporate purposes. During the three months ended August 31, 2018, we issued 1,368,250 four-year warrants at an exercise price of $0.50 per share at a fair value of $941,972 to the investors in the WestPark Offering as a penalty for failure to timely file a registration statement with respect to the securities we sold to them in the WestPark Offering.
The Canaccord Special Warrant Offering
On June 20, 2018, we executed an agency agreement with Canaccord Genuity Corp. and closed on a private offering of our Special Warrants for aggregate gross proceeds of CD$13,037,859 (USD$9,785,978). In connection therewith, we also entered into a Special Warrant Indenture and a Warrant Indenture with Odyssey Trust Company, as special warrant agent and warrant agent.
Pursuant to the offering, we issued 28,973,014 special warrants at a price of CD$0.45 (USD$0.34) per Special Warrant. Each Special Warrant was automatically exercised, for no additional consideration, into Units on November 30, 2018.
Each Unit consisted of one Unit Share and one Warrant to purchase one share of Common Stock. Each Warrant was to be exercisable at a price of CD$0.65 for three years after our Common Stock was listed on a recognized Canadian stock exchange, subject to adjustment in certain events. Because we did not receive a receipt from the applicable Canadian securities authorities for the qualifying prospectus by August 20, 2018, each Special Warrant entitled the holder to receive 1.1 Units (instead of one (1) Unit); provided, however, that any fractional entitlement to Penalty Units was rounded down to the nearest whole Penalty Unit. All Special Warrants were automatically exercised on November 30, 2018.
In connection with the Special Warrant Offering, we paid a cash commission and other fees equal to CD$1,413,267 (USD$1,060,773), a corporate finance fee equal to 1,448,651 Special Warrants with a fair value of USD$1,413,300, and 2,317,842 Broker Warrants. Each Broker Warrant entitles the holder thereof to acquire one unit at a price of CD$0.45 per unit for a period of 36 months from the date that our Common Stock is listed on a recognized Canadian stock exchange, subject to adjustment in certain events. Our Common Stock commenced trading on the Canadian Stock Exchange on January 7, 2019. During the three months ended August 31, 2018, we also issued investors 3,042,167 Special Warrants with a fair value of $7,142,550 as a penalty for failure to timely effect a Canadian prospectus with regard to the securities underlying the Special Warrants.
We used the proceeds for the Canaccord Special Warrant Offering to close the purchase of Oasis and for general corporate purposes.
The Navy Capital Investors
Effective July 31, 2018, we entered into a subscription agreement with Navy Capital Green International, Ltd., a British Virgin Islands limited company (“Navy Capital”), pursuant to which we agreed to sell to Navy Capital, for a purchase price of $3,000,000, 7,500,000 Units ($0.40 per unit), representing (i) 7,500,000 shares of our Common Stock, and (ii) three-year warrants to purchase an aggregate of 7,500,000 shares of our Common Stock (the “Navy Warrant Shares”) at an exercise price of $0.60 per share of Common Stock. We valued the warrants using the Black-Scholes valuation model, and allocated gross proceeds in the amount of $1,913,992 to the common stock and $1,086,008 to the warrants. The closing occurred on August 6, 2018. In the subscription agreement, we also agreed to file, on or before November 1, 2018, a registration statement with the SEC registering the shares of Common Stock and Navy Warrant Shares issued to Navy Capital. If we fail to file the registration statement on or before that date, we must issue to Navy Capital an additional number of units equal to ten percent (10%) of the units originally subscribed for by Navy Capital (which will include additional warrants at the original exercise price). The warrant is exercisable from time to time, in whole or in part for three years. The warrant has anti-dilution provisions that provide for an adjustment to the exercise price in the event of a future sale of Common Stock at a lower price, subject to certain exceptions as set forth in the warrant. The warrant also provides that it is callable at any time after the bid price of our Common Stock exceeds 120% of the exercise price of the warrant for a period of 20 consecutive business days.
Between August 8, 2018 and August 10, 2018, we entered into five subscription agreements, pursuant to which we sold, for an aggregate purchase price of $2,750,000, 6,875,000 Units ($0.40 per unit), representing (i) 6,875,000 shares of our Common Stock, and (ii) three-year warrants to purchase an aggregate of 6,875,000 shares of our Common Stock at an exercise price of $0.60 per share of Common Stock. We valued the warrants using the Black-Scholes valuation model, and allocated gross proceeds in the amount of $1,670,650 to the common stock and $1,079,350 to the warrants. The balance of the terms set forth in the subscription agreements are the same as the terms in the Navy Capital subscription agreement summarized above.
The Company valued warrants using the Black-Scholes valuation model utilizing the following variables:
|
May 31, |
May 31, |
|||||||
|
2019 |
2018 |
|||||||
|
Volatility |
79.02 to 400.3 |
% |
99.36 to 99.53 |
% |
||||
|
Dividends |
$ | 0 | $ | 0 | ||||
|
Risk-free interest rates |
2.68% to 2.77 |
% |
2.52% to 2.65 |
% |
||||
|
Term (years) |
3 | 3 | ||||||
We used the proceeds of the Navy Capital offering to fund a portion of our Expansion Plan and for general corporate purposes.
Liquidity and Capital Needs
Over the next twelve months we will likely require additional capital to cover our projected corporate level cash flow deficits, the implementation of our business plan, including the expansion of our Nevada operation, and the development of other revenue sources, including the closing of the IGH Option Agreement.
During the next twelve months we expect to complete Phase 1 and possibly Phase 2 of our Expansion Plan, which includes the buildout of our processing facility that will utilize our patented technology (Phase 1) and the substantial expansion of our grow facility in Nevada (Phase 2). We do not require additional financing to complete Phase 1 of our Expansion Plan. We hold an option to purchase IGH for a purchase price that includes $35 million in cash, which we plan to fund with the proceeds of future equity sales, warrant exercise proceeds and/or loans. Although we believe we will be successful in raising the capital required to close this acquisition, we have not entered into any definitive agreements with respect to such fundraising and there can be no assurances that we will be able to raise the necessary funds. We may also pursue additional acquisitions in the next twelve months but we have not entered into any definitive agreements with respect to either additional acquisitions or the capital necessary to finance them.
Although our revenues are expected to grow as we expand our operations, our revenues only recently exceeded our Oasis and City Trees operating costs and we do not yet exceed our Oasis and City Trees operating costs and corporate overhead. Although we believe we have funds sufficient to sustain our operations at their current level, if we require additional cash, we expect to obtain the necessary funds as described above; however, our prospects must be considered in light of the risks, expenses and difficulties frequently encountered by companies in their early stage of operations. To address these risks, we must, among other things, seek growth opportunities through additional debt and/or equity investments and acquisitions in our industry, successfully execute our business strategy, including our planned expansion and acquisitions, and successfully navigate any changes that may arise in the cannabis regulatory environment. We cannot assure that we will be successful in addressing such risks, and the failure to do so could have a material adverse effect on our business prospects, financial condition and results of operations.
Oasis Cannabis Transaction
On December 4, 2017, we entered into the Acquisition Agreement, with Alternative Solutions for us to acquire all of the outstanding equity interests in Alternative Solutions and the Oasis LLCs. Pursuant to the Acquisition Agreement, we paid a non-refundable deposit of $250,000 upon signing, which was followed by an additional payment of $1,800,000 approximately 45 days thereafter and were to receive, upon receipt of applicable regulatory approvals, an initial 10% of each of the Oasis LLCs. Regulatory approvals were received and the 10% membership interests were transferred to us.
On June 27, 2018, we closed on the purchase of the remaining 90% of the membership interests in Alternative Solutions and the Oasis LLCs from the owners thereof (excluding Alternative Solutions). The closing consideration was as follows: $5,995,543 in cash, a $4.0 million promissory note due in December 2019, known as the Oasis Note, and $6,000,000 in shares of our Common Stock. The cash payment of $5,995,543 was less than the $6,200,000 payment originally contemplated because the Company assumed an additional $204,457 of liabilities.
The number of shares to be issued was computed as follows: $6,000,000 divided by the lower of $1.00 or the conversion price to receive one share of our Common Stock in our first equity offering of a certain minimum size that commenced in 2018, multiplied by 80%. This price was determined to be $0.272 per share. The Oasis Note is secured by a first priority security interest over our membership interests in Alternative Solutions and the Oasis LLCs, and by the assets of each of the Oasis LLCs and Alternative Solutions. We also delivered a confession of judgment to a representative of the former owners of Alternative Solutions and the Oasis LLCs (other than Alternative Solutions) that will generally become effective in the event of any event of default under the Oasis Note.
At the time of closing of the Acquisition Agreement, Alternative Solutions owed certain amounts to a consultant known as 4Front Advisors, which amount was in dispute. In August 2019, we made a payment to this company to settle this dispute and the Oasis Note was reduced accordingly.
In May 2020, the former owners of Alternative Solutions and the Oasis LLCs (other than Alternative Solutions) will also be entitled to a $1,000,000 payment from us if the existing dispensary operated by an Oasis LLC has maintained an average revenue of $20,000 per day during the 2019 calendar year.
The transfer of 90% of the membership interests in Alternative Solutions and the Oasis LLCs to us was approved by the State of Nevada on December 12, 2018.
Consulting Agreements
We periodically use the services of outside investor relations consultants. During the year ended May 31, 2016, pursuant to a consulting agreement, we agreed to issue 10,000 shares of Common Stock per month, valued at $11,600 per month, to a consultant in exchange for investor relations consulting services. The consulting agreement was terminated during the first month of its term. The parties are in discussions regarding whether any shares of our Common Stock have been earned and it is uncertain whether any shares will be issued. As of May 31, 2018, we have included 20,000 shares of Common Stock, valued at $23,200 in stock payable on the accompanying balance sheets. The shares were valued based on the closing market price on the grant date.
On December 29, 2015, pursuant to a consulting agreement, we agreed to issue 25,000 shares of Common Stock per month, valued at $21,250, to a consultant in exchange for investor relations consulting services. The consulting agreement was terminated during the first month of its term. The parties are in discussions regarding whether any shares of our Common Stock have been earned and it is uncertain whether any shares will be issued. As of May 31, 2018, we had 50,000 shares of Common Stock, valued at $42,500 included in stock payable on the accompanying balance sheet. The shares were valued based on the closing market price on the grant date.
In June 2017, we entered into a letter agreement to amend our September 22, 2014 Investor Relations Consulting Agreement. Pursuant to the amendment, we agreed to issue the consultant 24,000 shares of our restricted Common Stock to satisfy $6,000 of past due invoices for services previously rendered by the consultant from January 2017 through June 2017.
On March 2, 2018, we issued 350,000 shares of Common Stock to a consultant pursuant to the terms of a consulting agreement for investor relations services. The shares were valued on the date of grant at $261,800.
On July 24, 2018, we issued 700,000 shares of Common Stock with a fair value of $490,000 to Star Associates for services in connection with the Oasis acquisition. Star Associates is controlled by Andrew Glashow, a director (and current officer) of the Company.
On September 11, 2018, the Company issued 31,250 shares of common stock with a fair value of $25,310 in exchange for legal services previously rendered to the Company. These shares were accrued on February 8, 2018, and were issued from stock payable.
On August 16, 2019, we amended a consulting agreement whereby we agreed to issue up to 200,000 shares of common stock plus pay certain amounts in exchange for the consultant’s development for us of a corporate finance and investor relations campaign, which services will be provided over a six month period.
Going Concern
Our financial statements were prepared using accounting principles generally accepted in the United States of America applicable to a going concern, which contemplate the realization of assets and liquidation of liabilities in the normal course of business. We have incurred continuous losses from operations since inception, and have an accumulated deficit of $46,188,151 as of May 31, 2019, compared to $18,569,094, as of May 31, 2018. That said, we had working capital of $5,753,023 as of May 31, 2019, compared to a working capital deficit of $2,634,774 at May 31, 2018. The report of our independent auditors for the year ended May 31, 2019, however, contained a going concern qualification. Our ability to continue as a going concern must be considered in light of the problems, expenses, and complications frequently encountered by early stage companies.
Our ability to continue as a going concern is dependent on our ability to generate sufficient cash from operations to meet our cash needs, to borrow capital and to sell equity to support our plans to acquire operating businesses, open processing facilities and finance ongoing operations. There can be no assurance, however, that we will be successful in our efforts to raise additional debt or equity capital and/or that cash generated by our future operations will be adequate to meet our needs. These factors, among others, indicate that we may be unable to continue as a going concern for a reasonable period of time.
Off-Balance Sheet Arrangements
We do not have any off-balance sheet arrangements that have, or are reasonably likely to have, a current or future effect on our financial condition, changes in financial condition, revenues or expenses, results of operations, liquidity, capital expenditures or capital resources that are material to investors.
Recently Issued Accounting Standards
Accounting standards promulgated by the Financial Accounting Standards Board (the “FASB”) are subject to change. Changes in such standards may have an impact on our future financial statements. The following are a summary of recent accounting developments.
In May 2014, the FASB issued Accounting Standards Update (“ASU”) 2014-09, Revenue from Contracts with Customers (Topic 606), to clarify the principles of recognizing revenue and create common revenue recognition guidance between U.S. GAAP and International Financial Reporting Standards. Under ASU 2014-09, revenue is recognized when a customer obtains control of promised goods or services and is recognized at an amount that reflects the consideration expected to be received in exchange for such goods or services. In addition, ASU 2014-09 requires disclosure of the nature, amount, timing, and uncertainty of revenue and cash flows arising from contracts with customers. The ASU is effective for fiscal years beginning after December 15, 2017. The new revenue standard is principle-based and interpretation of those principles may vary from company to company based on their unique circumstances. It is possible that interpretation, industry practice, and guidance may evolve as companies and the accounting profession work to implement this new standard. The implementation of this standard did not have a material effect on our results of operations.
In February 2016, the FASB issued ASU No. 2016-02, Leases (Topic 842): Accounting for Leases. This update requires that lessees recognize right-of-use assets and lease liabilities that are measured at the present value of the future lease payments at lease commencement date. The recognition, measurement, and presentation of expenses and cash flows arising from a lease by a lessee will largely remain unchanged and shall continue to depend on its classification as a finance or operating lease. We have performed a comprehensive review in order to determine what changes were required to support the adoption of this new standard. We adopted the ASU and related amendments on June 1, 2019 and expect to elect certain practical expedients permitted under the transition guidance. We elected the optional transition method that allows for a cumulative-effect adjustment in the period of adoption and will not restate prior periods. Under the new guidance, the majority of our leases will continue to be classified as operating leases. During the first quarter of fiscal 2020, we will complete our implementation of our processes and policies to support the new lease accounting and reporting requirements. Based on our lease portfolio as of June 1, 2019, we preliminarily estimate the impact of the adoption of ASU 2016-02 to increase both our total assets and total liabilities in the range of $850,000 to $1,050,000. The adoption of this ASU is not expected to have a significant impact on our consolidated statements of operations or cash flows. We continue to finalize the implementation of the new processes and the assessment of the impact of this adoption on our consolidated financial statements; therefore, the preliminary estimated impacts disclosed can change, and the final impact will be known once the adoption is completed during the first quarter of fiscal 2020.
In August 2016, the FASB issued ASU 2016-15, Statement of Cash Flows (Topic 230). The update addresses eight specific cash flow issues and is intended to reduce diversity in practice in how certain cash receipts and cash payments are presented and classified in the statement of cash flows. This update is effective for reporting periods beginning after December 15, 2017, including interim periods within the reporting period. Adoption of ASU 2016-15 did not have a material effect on our financial statements.
In January 2017, the FASB issued ASU No. 2017-04, Simplifying the Test for Goodwill Impairment, which simplifies the subsequent measurement of goodwill by eliminating Step 2 from the goodwill impairment test. In computing the implied fair value of goodwill under Step 2, current U.S. GAAP requires the performance of procedures to determine the fair value at the impairment testing date of assets and liabilities (including unrecognized assets and liabilities) following the procedure that would be required in determining the fair value of assets acquired and liabilities assumed in a business combination. Instead, the amendments under this ASU require the goodwill impairment test to be performed by comparing the fair value of a reporting unit with its carrying amount. An impairment charge should be recognized for the amount by which the carrying amount exceeds the reporting unit’s fair value; however, the loss recognized should not exceed the total amount of goodwill allocated to that reporting unit. The ASU becomes effective for us on January 1, 2020. The amendments in this ASU will be applied on a prospective basis. Early adoption is permitted for interim or annual goodwill impairment tests performed.
In May 2017, the FASB issued ASU No. 2017-09, Stock Compensation - Scope of Modification Accounting, which provides guidance on which changes to the terms or conditions of a share-based payment award require an entity to apply modification accounting. The ASU requires that an entity account for the effects of a modification unless the fair value (or calculated value or intrinsic value, if used), vesting conditions and classification (as equity or liability) of the modified award are all the same as for the original award immediately before the modification. The ASU became effective for us on January 1, 2018, and will be applied to an award modified on or after the adoption date. Adoption of ASU 2017-09 did not have a material effect on our financial statements.
Effective June 1, 2018, we adopted Accounting Standards Codification (“ASC”) 606 — Revenue from Contracts with Customers. Under ASC 606, we recognize revenue from the commercial sales of products, licensing agreements and contracts to perform pilot studies by applying the following steps: (1) identify the contract with a customer; (2) identify the performance obligations in the contract; (3) determine the transaction price; (4) allocate the transaction price to each performance obligation in the contract; and (5) recognize revenue when each performance obligation is satisfied. For the comparative periods, revenue has not been adjusted and continues to be reported under ASC 605 — Revenue Recognition. Under ASC 605, revenue is recognized when the following criteria are met: (1) persuasive evidence of an arrangement exists; (2) the performance of service has been rendered to a customer or delivery has occurred; (3) the amount of fee to be paid by a customer is fixed and determinable; and (4) the collectability of the fee is reasonably assured. There was no impact on our financial statements as a result of adopting ASC 606.
On June 1, 2018, we adopted ASU 2017-11 and accordingly reclassified the fair value of the reset provisions embedded in convertible notes payable and certain warrants with embedded anti-dilutive provisions from liability to equity in the aggregate amount of $1,265,751.
There are various other updates recently issued, most of which represented technical corrections to the accounting literature or application to specific industries and are not expected to a have a material impact on our consolidated financial position, results of operations or cash flows.
Critical Accounting Estimates
Management uses various estimates and assumptions in preparing our financial statements in accordance with generally accepted accounting principles. These estimates and assumptions affect the reported amounts of assets and liabilities, the disclosure of contingent assets and liabilities, and the reported revenues and expenses. Accounting estimates that are the most important to the presentation of our results of operations and financial condition, and which require the greatest use of judgment by management, are designated as our critical accounting estimates. We have the following critical accounting estimates:
|
● |
Estimates and assumptions used in the valuation of derivative liabilities: Management utilizes a lattice model to estimate the fair value of derivative liabilities. The model includes subjective assumptions that can materially affect the fair value estimates. |
Item 7A. Quantitative and Qualitative Disclosure about Market Risk.
This item is not applicable as we are currently considered a smaller reporting company.
Item 8. Financial Statements and Supplementary Data.
INDEX TO FINANCIAL STATEMENTS
|
|
Page |
|
|
|
|
Financial Statements |
|
|
|
|
|
Report of Independent Registered Public Accounting Firm |
F-1 |
|
|
|
|
Consolidated Balance Sheets |
F-2 |
|
|
|
|
Consolidated Statements of Operations |
F-3 |
|
|
|
|
Consolidated Statement of Changes in Stockholders’ Equity (Deficit) |
F-4 |
|
|
|
|
Consolidated Statements of Cash Flows |
F-5 |
|
|
|
|
Consolidated Notes to Financial Statements |
F-6 |

REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
To the Board of Directors and
Stockholders of CLS Holdings USA, Inc.
Opinion on the Financial Statements
We have audited the accompanying consolidated balance sheets of CLS Holdings USA, Inc. (the Company) as of May 31, 2019 and 2018, and the related consolidated statements of operations, stockholders’ equity (deficit), and cash flows for each of the years in the two-year period ended May 31, 2019, and the related notes and schedules (collectively referred to as the financial statements). In our opinion, the financial statements present fairly, in all material respects, the financial position of the Company as of May 31, 2019 and 2018, and the results of its operations and its cash flows for each of the years in the two-year period ended May 31, 2019, in conformity with accounting principles generally accepted in the United States of America.
Basis for Opinion
These financial statements are the responsibility of the Company’s management. Our responsibility is to express an opinion on the Company’s financial statements based on our audits. We are a public accounting firm registered with the Public Company Accounting Oversight Board (United States) (PCAOB) and are required to be independent with respect to the Company in accordance with the U.S. federal securities laws and the applicable rules and regulations of the Securities and Exchange Commission and the PCAOB.
We conducted our audits in accordance with the standards of the PCAOB. Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement, whether due to error or fraud. The Company is not required to have, nor were we engaged to perform, an audit of its internal control over financial reporting. As part of our audits, we are required to obtain an understanding of internal control over financial reporting, but not for the purpose of expressing an opinion on the effectiveness of the Company’s internal control over financial reporting. Accordingly, we express no such opinion.
Our audits included performing procedures to assess the risks of material misstatement of the financial statements, whether due to error or fraud, and performing procedures that respond to those risks. Such procedures included examining, on a test basis, evidence regarding the amounts and disclosures in the financial statements. Our audits also included evaluating the accounting principles used and significant estimates made by management, as well as evaluating the overall presentation of the financial statements. We believe that our audits provide a reasonable basis for our opinion.
The accompanying financial statements have been prepared assuming that the Company will continue as a going concern. As discussed in Note 2 to the financial statements, the Company suffered a net loss from operations and has a net capital deficiency, which raises substantial doubt about its ability to continue as a going concern. Management’s plans regarding those matters are described in Note 2. The financial statements do not include any adjustments that might result from the outcome of this uncertainty.
/s/ M&K CPAS, PLLC.
We have served as the Company’s auditor since 2011.
Houston, TX
August 29, 2019
CLS Holdings USA, Inc.
|
May 31, |
May 31, |
|||||||
|
2019 |
2018 |
|||||||
|
ASSETS |
||||||||
|
Current assets |
||||||||
|
Cash and cash equivalents |
$ | 10,525,791 | $ | 52,964 | ||||
|
Accounts Receivable |
163,571 | - | ||||||
|
Inventory |
746,833 | - | ||||||
|
Prepaid expenses and other current assets |
390,413 | 1,410 | ||||||
|
Notes receivable - current portion |
850,958 | - | ||||||
|
Total current assets |
12,677,566 | 54,374 | ||||||
|
Investment |
2,709 | 2,050,000 | ||||||
|
Note receivable |
4,299,042 | - | ||||||
|
Interest receivable |
178,258 | - | ||||||
|
Property, plant and equipment, net of accumulated depreciation of $546,408 and $2,674, respectively |
1,910,301 | - | ||||||
|
Intangible assets, net of accumulated amortization of $116,476 and $1,402, respectively |
1,525,087 | 898 | ||||||
|
Goodwill |
25,742,899 | - | ||||||
|
Other assets |
167,455 | - | ||||||
|
Total assets |
$ | 46,503,317 | $ | 2,105,272 | ||||
|
LIABILITIES AND STOCKHOLDERS' EQUITY (DEFICIT) |
||||||||
|
Current liabilities |
||||||||
|
Accounts payable and accrued liabilities |
$ | 1,517,127 | $ | 826,621 | ||||
|
Accrued compensation, related party |
- | 120,417 | ||||||
|
Due to related party |
- | 17,930 | ||||||
|
Accrued interest |
474,800 | 24,748 | ||||||
|
Accrued interest, related party |
- | 5,143 | ||||||
|
Notes payable, net of discount of $67,384 and $0, respectively |
3,932,616 | 310,000 | ||||||
|
Notes payable, related parties |
- | 75,137 | ||||||
|
Convertible notes payable, net of discount of $0 and $561,599, respectively |
- | 43,401 | ||||||
|
Contingent liability |
1,000,000 | - | ||||||
|
Derivative liability |
- | 1,265,751 | ||||||
|
Total current liabilities |
6,924,543 | 2,689,148 | ||||||
|
Noncurrent liabilities |
||||||||
|
Convertible notes payable - Long Term, net of discount of $3,819,010 and $733,928, respectively |
14,541,220 | 41,072 | ||||||
|
Convertible notes payable, related parties, net of discount of $0 and $65,918, respectively |
- | 2,832 | ||||||
|
Total Liabilities |
21,465,763 | 2,733,052 | ||||||
|
Commitments and contingencies |
- | - | ||||||
|
Stockholder's equity (deficit) |
||||||||
|
Preferred stock, $0.001 par value; 20,000,000 shares authorized; no shares issued |
- | - | ||||||
|
Common stock, $0.0001 par value; 750,000,000 and 250,000,000 shares authorized at May 31, 2019 and 2018, respectively; 125,839,095 and 50,128,972 shares issued and outstanding at May 31, 2019 and 2018, respectively |
12,585 | 5,013 | ||||||
|
Additional paid-in capital |
70,758,025 | 17,628,717 | ||||||
|
Common stock subscribed |
455,095 | 307,584 | ||||||
|
Accumulated deficit |
(46,188,151 |
) |
(18,569,094 |
) |
||||
|
Total stockholder's equity (deficit) |
25,037,554 | (627,780 |
) |
|||||
|
Total liabilities and stockholders' equity (deficit) |
$ | 46,503,317 | $ | 2,105,272 | ||||
See notes to consolidated financial statements.
CLS Holdings USA, Inc.
Consolidated Statements of Operations
|
For the |
For the |
|||||||
|
Year Ended |
Year Ended |
|||||||
|
May 31, 2019 |
May 31, 2018 |
|||||||
|
Revenue |
$ | 8,459,048 | $ | - | ||||
|
Cost of goods sold |
4,836,166 | - | ||||||
|
Gross margin |
3,622,882 | - | ||||||
|
Selling, general and administrative expenses |
26,472,057 | 3,116,040 | ||||||
|
Total operating expenses |
26,472,057 | 3,116,040 | ||||||
|
Operating loss |
(22,849,175 |
) |
(3,116,040 |
) |
||||
|
Other (income) expense: |
||||||||
|
Interest expense, net |
4,447,993 | 4,709,940 | ||||||
|
Gain on settlement of debt |
- | (3,480 |
) |
|||||
|
Loss on revaluation of contingent liability |
321,889 | - | ||||||
|
Loss on modification of debt |
- | 29,145 | ||||||
|
Loss on note exchange |
- | 404,082 | ||||||
|
Loss on extinguishment of debt |
- | 989,032 | ||||||
|
Prepayment penalty |
- | 137,000 | ||||||
|
Change in fair value of derivative |
- | 195,725 | ||||||
|
Total other expense |
4,769,882 | 6,461,444 | ||||||
|
Income (Loss) before income taxes |
(27,619,057 |
) |
(9,577,484 |
) |
||||
|
Income tax expense |
- | - | ||||||
|
Net income (loss) |
$ | (27,619,057 |
) |
$ | (9,577,484 |
) |
||
|
Net income (loss) per share - basic |
$ | (0.27 |
) |
$ | (0.24 |
) |
||
|
Net income (loss) per share - diluted |
$ | (0.27 |
) |
$ | (0.24 |
) |
||
|
Weighted average shares outstanding - basic |
102,869,612 | 39,224,613 | ||||||
|
Weighted average shares outstanding - diluted |
102,869,612 | 39,224,613 | ||||||
See notes to consolidated financial statements.
CLS Holdings USA, Inc.
Consolidated Statements of Stockholders' Equity (Deficit)
|
|
|
|
|
|
|
|
|
|
|
Additional |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Common Stock |
|
|
Paid In |
|
|
Stock |
|
|
Accumulated |
|
|
|
|
|
||||||||
|
|
|
Amount |
|
|
Value |
|
|
Capital |
|
|
Payable |
|
|
Deficit |
|
|
Total |
|
||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Balance, May 31, 2017 |
|
|
32,852,944 |
|
|
$ |
3,286 |
|
|
$ |
7,032,836 |
|
|
$ |
68,950 |
|
|
$ |
(8,991,610 |
) |
|
$ |
(1,886,538 |
) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Common stock issued to consultant for services |
|
|
374,000 |
|
|
|
37 |
|
|
|
264,283 |
|
|
|
25,313 |
|
|
|
- |
|
|
|
289,633 |
|
|
Common stock issued for debt exchange |
|
|
6,000,000 |
|
|
|
600 |
|
|
|
2,353,437 |
|
|
|
- |
|
|
|
- |
|
|
|
2,354,037 |
|
|
Common stock issued as commitment fees |
|
|
250,000 |
|
|
|
25 |
|
|
|
94,975 |
|
|
|
- |
|
|
|
- |
|
|
|
95,000 |
|
|
Common stock issued to officer |
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
213,321 |
|
|
|
- |
|
|
|
213,321 |
|
|
Common stock issued for cash, net of issuance costs |
|
|
5,473,000 |
|
|
|
547 |
|
|
|
1,460,368 |
|
|
|
- |
|
|
|
- |
|
|
|
1,460,915 |
|
|
Common stock issued for conversion of debt |
|
|
5,179,028 |
|
|
|
518 |
|
|
|
1,617,928 |
|
|
|
- |
|
|
|
- |
|
|
|
1,618,446 |
|
|
Warrants issued with debt |
|
|
- |
|
|
|
- |
|
|
|
1,804,470 |
|
|
|
- |
|
|
|
- |
|
|
|
1,804,470 |
|
|
Settlement of derivative liability |
|
|
- |
|
|
|
- |
|
|
|
442,775 |
|
|
|
- |
|
|
|
- |
|
|
|
442,775 |
|
|
Placement agent warrants |
|
|
- |
|
|
|
- |
|
|
|
503,655 |
|
|
|
- |
|
|
|
- |
|
|
|
503,655 |
|
|
Warrants issued to consultants |
|
|
- |
|
|
|
- |
|
|
|
294,173 |
|
|
|
- |
|
|
|
- |
|
|
|
294,173 |
|
|
Discount on notes from beneficial conversion feature |
|
|
- |
|
|
|
- |
|
|
|
1,758,741 |
|
|
|
- |
|
|
|
- |
|
|
|
1,758,741 |
|
|
Imputed interest |
|
|
- |
|
|
|
- |
|
|
|
1,076 |
|
|
|
- |
|
|
|
- |
|
|
|
1,076 |
|
|
Net loss for the year ended May 31, 2018 |
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
(9,577,484 |
) |
|
|
(9,577,484 |
) |
|
Balance, May 31, 2018 |
|
|
50,128,972 |
|
|
$ |
5,013 |
|
|
$ |
17,628,717 |
|
|
$ |
307,584 |
|
|
$ |
(18,569,094 |
) |
|
$ |
(627,780 |
) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Common stock issued for conversion of debt |
|
|
3,697,511 |
|
|
|
370 |
|
|
|
1,295,320 |
|
|
|
- |
|
|
|
- |
|
|
|
1,295,690 |
|
|
Common stock issued in connection with Oasis acquisition |
|
|
22,058,823 |
|
|
|
2,206 |
|
|
|
15,438,970 |
|
|
|
- |
|
|
|
- |
|
|
|
15,441,176 |
|
|
Common stock issued to consultant |
|
|
731,250 |
|
|
|
73 |
|
|
|
515,240 |
|
|
|
(25,313 |
) |
|
|
- |
|
|
|
490,000 |
|
|
Common stock issued to officer |
|
|
625,000 |
|
|
|
62 |
|
|
|
281,438 |
|
|
|
(230,820 |
) |
|
|
- |
|
|
|
50,680 |
|
|
Common stock to be issued to officer |
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
403,644 |
|
|
|
- |
|
|
|
403,644 |
|
|
Common stock issued for cash |
|
|
14,375,000 |
|
|
|
1,438 |
|
|
|
5,748,562 |
|
|
|
- |
|
|
|
- |
|
|
|
5,750,000 |
|
|
Special Warrants issued for cash |
|
|
- |
|
|
|
- |
|
|
|
9,785,978 |
|
|
|
- |
|
|
|
- |
|
|
|
9,785,978 |
|
|
Cashless exercise of warrant |
|
|
148,951 |
|
|
|
15 |
|
|
|
(15 |
) |
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
Warrant issued due to penalty |
|
|
- |
|
|
|
- |
|
|
|
941,972 |
|
|
|
- |
|
|
|
- |
|
|
|
941,972 |
|
|
Warrants issued as compensation for offering |
|
|
- |
|
|
|
- |
|
|
|
2,369,830 |
|
|
|
- |
|
|
|
- |
|
|
|
2,369,830 |
|
|
Units issued as compensation for offering |
|
|
559,750 |
|
|
|
56 |
|
|
|
557,279 |
|
|
|
|
|
|
|
|
|
|
|
557,335 |
|
|
Warrants issued to placement agent |
|
|
- |
|
|
|
- |
|
|
|
1,413,300 |
|
|
|
- |
|
|
|
- |
|
|
|
1,413,300 |
|
|
Special warrant issued due to penalty |
|
|
- |
|
|
|
- |
|
|
|
7,142,550 |
|
|
|
- |
|
|
|
- |
|
|
|
7,142,550 |
|
|
Common stock issued for exercise of special warrants |
|
|
33,463,838 |
|
|
|
3,347 |
|
|
|
(3,347 |
) |
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
Common stock shares issued for settlement |
|
|
50,000 |
|
|
|
5 |
|
|
|
47,495 |
|
|
|
- |
|
|
|
- |
|
|
|
47,500 |
|
|
Foreign currency transaction loss on equity offering |
|
|
- |
|
|
|
- |
|
|
|
403,588 |
|
|
|
- |
|
|
|
- |
|
|
|
403,588 |
|
|
Discount on notes from beneficial conversion feature |
|
|
- |
|
|
|
- |
|
|
|
5,888,707 |
|
|
|
- |
|
|
|
- |
|
|
|
5,888,707 |
|
|
Reclassification of derivative upon adoption of ASU 2017-11 |
|
|
- |
|
|
|
- |
|
|
|
1,265,751 |
|
|
|
- |
|
|
|
- |
|
|
|
1,265,751 |
|
|
Derivative valuation of reset event |
|
|
- |
|
|
|
- |
|
|
|
35,883 |
|
|
|
- |
|
|
|
- |
|
|
|
35,883 |
|
|
Imputed interest |
|
|
- |
|
|
|
- |
|
|
|
807 |
|
|
|
- |
|
|
|
- |
|
|
|
807 |
|
|
Net loss for the year ended May 31, 2019 |
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
(27,619,057 |
) |
|
|
(27,619,057 |
) |
|
Balance, May 31, 2019 |
|
|
125,839,095 |
|
|
$ |
12,585 |
|
|
$ |
70,758,025 |
|
|
$ |
455,095 |
|
|
$ |
(46,188,151 |
) |
|
$ |
25,037,554 |
|
See notes to consolidated financial statements.
CLS Holdings USA, Inc.
Consolidated Statements of Cash Flows
|
For the |
For the |
|||||||
|
Year Ended |
Year Ended |
|||||||
|
May 31, 2019 |
May 31, 2018 |
|||||||
|
CASH FLOWS FROM OPERATING ACTIVITIES |
||||||||
|
Net income (loss) |
$ | (27,619,057 |
) |
$ | (9,577,484 |
) |
||
|
Adjustments to reconcile net loss to net cash used in operating activities: |
||||||||
|
Imputed interest |
807 | 1,076 | ||||||
|
Excess derivative |
- | 1,940,439 | ||||||
|
Change in fair value of derivative |
- | 195,725 | ||||||
|
Loss on modification of debt |
- | 29,145 | ||||||
|
Stock-based compensation |
- | 794,607 | ||||||
|
Warrants issued to placement agent |
3,783,130 | 503,655 | ||||||
|
Loss on note exchange |
- | 404,082 | ||||||
|
Loss on extinguishment of debt |
- | 989,032 | ||||||
|
Gain on settlement of Account Payable |
- | (3,480 |
) |
|||||
|
Prepayment penalty |
- | 137,000 | ||||||
|
Revaluation of contingent liability |
321,889 | - | ||||||
|
Amortization of debt discounts |
3,576,161 | 2,534,103 | ||||||
|
Warrants and Special Warrants issued due to penalty |
8,084,522 | - | ||||||
|
Units issued to placement agent |
557,335 | - | ||||||
|
Non-cash offering costs of equity financing |
403,588 | - | ||||||
|
Fair value of shares vested by officers |
454,324 | - | ||||||
|
Fair value of shares issued to consultants |
490,000 | - | ||||||
|
Fair value of shares issued in settlement |
47,500 | - | ||||||
|
Depreciation and amortization expense |
288,351 | 1,322 | ||||||
|
Expense from derivative triggering event |
12,659 | - | ||||||
|
Changes in assets and liabilities: |
||||||||
|
Other assets |
- | 50,000 | ||||||
|
Accounts payable and accrued expenses |
(484,609 |
) |
300,421 | |||||
|
Accrued compensation |
(120,417 |
) |
216,667 | |||||
|
Accrued interest, related party |
(362 |
) |
96,211 | |||||
|
Deferred rent |
1,667 | (49,565 |
) |
|||||
|
Accrued interest |
995,941 | 11,746 | ||||||
|
Accounts receivable |
(128,134 |
) |
- | |||||
|
Interest receivable |
(178,258 |
) |
- | |||||
|
Inventory |
(340,880 |
) |
- | |||||
|
Prepaid expenses |
(292,769 |
) |
- | |||||
|
Due to related parties |
(17,930 |
) |
- | |||||
|
Net cash used in operating activities |
(10,164,542 |
) |
(1,425,298 |
) |
||||
|
CASH FLOWS FROM INVESTING ACTIVITIES |
||||||||
|
Payments to purchase property, plant and equipment |
(1,037,262 |
) |
- | |||||
|
Payments to acquire note receivable |
(5,150,000 |
) |
- | |||||
|
Payment for investment in Alternative Solutions, net of cash received of $14,612 |
(5,982,710 |
) |
(2,050,000 |
) |
||||
|
Net cash used in investing activities |
(12,169,972 |
) |
(2,050,000 |
) |
||||
|
CASH FLOWS FROM FINANCING ACTIVITIES |
||||||||
|
Proceeds from related party convertible notes payable |
- | 761,829 | ||||||
|
Proceeds from notes payable |
- | 410,000 | ||||||
|
Proceeds from convertible notes payable |
18,369,000 | 1,655,000 | ||||||
|
Principal payments on notes payable |
(1,060,000 |
) |
(100,000 |
) |
||||
|
Principal payments on related party notes payable |
(137 |
) |
(237,794 |
) |
||||
|
Principal payments on convertible notes payable |
(37,500 |
) |
(500,000 |
) |
||||
|
Proceeds from sale of equity |
15,535,978 | 1,460,917 | ||||||
|
Net cash provided by financing activities |
32,807,341 | 3,449,952 | ||||||
|
Net increase in cash and cash equivalents |
10,472,827 | (25,346 |
) |
|||||
|
Cash and cash equivalents at beginning of period |
52,964 | 78,310 | ||||||
|
Cash and cash equivalents at end of period |
$ | 10,525,791 | $ | 52,964 | ||||
|
SUPPLEMENTAL DISCLOSURE OF CASH FLOW INFORMATION: |
||||||||
|
Interest paid |
$ | 8,964 | $ | - | ||||
|
Income taxes paid |
$ | - | $ | - | ||||
|
NON-CASH INVESTING AND FINANCING ACTIVITIES: |
||||||||
|
Convertible note issued for unpaid accrued salary |
$ | - | $ | 150,000 | ||||
|
Discount on notes due to derivatives |
$ | - | $ | 1,758,741 | ||||
|
Related party notes payable reclassified as related party convertible notes payable |
$ | - | $ | 1,116,856 | ||||
|
Common stock issued for conversion of related party notes payable |
$ | - | $ | 2,023,666 | ||||
|
Common stock issued for conversion of convertible notes payable |
$ | - | $ | 2,554,924 | ||||
|
Common stock issued for settlement of accounts payable |
$ | - | $ | 6,000 | ||||
|
Settlement of derivative liability |
$ | - | $ | 442,775 | ||||
|
Common stock issued for services, previously accrued |
$ | 17,500 | $ | - | ||||
|
Accrued interest capitalized to principal of notes payable |
$ | 491,230 | $ | - | ||||
|
Convertible note issued for unpaid accrued salary |
$ | 75,000 | $ | - | ||||
|
Beneficial conversion feature on convertible notes |
$ | 5,888,707 | $ | - | ||||
|
Note payable exchanged for common stock |
$ | 1,295,690 | $ | - | ||||
|
Charge to paid-in capital for par value of shares issued in cashless exercise of warrants |
$ | 3,362 | $ | - | ||||
|
Reclassify derivative liability to paid-in capital upon adoption of ASU 2017-11 |
$ | 1,265,751 | $ | - | ||||
|
Shares issued for services from stock payable |
$ | 25,313 | $ | - | ||||
See notes to consolidated financial statements.
CLS HOLDINGS USA, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
NOTE 1 – BUSINESS ORGANIZATION AND NATURE OF OPERATIONS
CLS Holdings USA, Inc. (the “Company”) was originally incorporated as Adelt Design, Inc. (“Adelt”) on March 31, 2011 to manufacture and market carpet binding art. Production and marketing of carpet binding art never commenced.
On November 12, 2014, CLS Labs, Inc. (“CLS Labs”) acquired 10,000,000 shares, or 55.6%, of the outstanding shares of common stock of Adelt from its founder, Larry Adelt. On that date, Jeffrey Binder, the Chairman, President and Chief Executive Officer of CLS Labs, was appointed Chairman, President and Chief Executive Officer of the Company. On November 20, 2014, Adelt adopted amended and restated articles of incorporation, thereby changing its name to CLS Holdings USA, Inc. Effective December 10, 2014, the Company effected a reverse stock split of its issued and outstanding common stock at a ratio of 1-for-0.625 (the “Reverse Split”), wherein 0.625 shares of the Company’s common stock were issued in exchange for each share of common stock issued and outstanding. As a result, 6,250,000 shares of the Company’s common stock were issued to CLS Labs in exchange for the 10,000,000 shares that it owned by virtue of the above-referenced purchase from Larry Adelt.
On April 29, 2015, the Company, CLS Labs and CLS Merger Inc., a Nevada corporation and wholly owned subsidiary of CLS Holdings (“Merger Sub”), entered into an Agreement and Plan of Merger (the “Merger Agreement”) and completed a merger, whereby CLS Merger Inc. merged with and into CLS Labs, with CLS Labs remaining as the surviving entity (the “Merger”). Upon the consummation of the Merger, the shares of the common stock of CLS Holdings owned by CLS Labs were extinguished and the former stockholders of CLS Labs were issued an aggregate of 15,000,000 (post Reverse Split) shares of common stock in CLS Holdings in exchange for their shares of common stock in CLS Labs. As a result of the Merger, the Company acquired the business of CLS Labs and abandoned its previous business.
The Company has been issued a U.S. patent with respect to its proprietary method of extracting cannabinoids from cannabis plants and converting the resulting cannabinoid extracts into concentrates such as oils, waxes, edibles and shatter. These concentrates may be ingested in a number of ways, including through vaporization via electronic cigarettes (“e-cigarettes”), and used for a variety of pharmaceutical and other purposes. Internal testing of this extraction method and conversion process has revealed that it produces a cleaner, higher quality product and a significantly higher yield than the cannabinoid extraction processes currently existing in the marketplace. The Company has not commercialized its patented proprietary process or otherwise earned any revenues from it. The Company plans to generate revenues through licensing, fee-for-service and joint venture arrangements related to its patented proprietary method of extracting cannabinoids from cannabis plants and converting the resulting cannabinoid extracts into saleable concentrates.
On December 4, 2017, the Company and Alternative Solutions, entered into a Membership Interest Purchase Agreement (the “Acquisition Agreement”), as amended, for the Company to acquire the Oasis LLCs from Alternative Solutions. Pursuant to the Acquisition Agreement, the Company initially contemplated acquiring all of the membership interests in the Oasis LLCs from Alternative Solutions. Just prior to closing, the parties agreed that the Company would instead acquire all of the membership interests in Alternative Solutions, the parent of the Oasis LLCs, from its members, and the membership interests in the Oasis LLCs owned by members other than Alternative Solutions.
Pursuant to the Acquisition Agreement, the Company paid a non-refundable deposit of $250,000 upon signing, which was followed by an additional payment of $1,800,000 paid in February 2018, for an initial 10% of each of the Oasis LLCs. At that time, the Company applied for regulatory approval to own an interest in the Oasis LLCs, which approval was received. On June 27, 2018, the Company made the payments to indirectly acquire the remaining 90% of the Oasis LLCs, which were equal to cash in the amount of $5,995,543, a $4.0 million promissory note due in December 2019 (the “Oasis Note”), and 22,058,823 shares of its common stock (the “Purchase Price Shares”) (collectively, the “Closing Consideration”). The cash payment of $5,995,543 was less than the $6,200,000 payment originally contemplated because the Company assumed an additional $204,457 of liabilities. The Company used the proceeds of a Canadian private securities offering to fund the cash portion of the Closing Consideration. The Company then applied for regulatory approval to own the additional 90% in membership interests in the Oasis LLCs, which it received on December 12, 2018.
On January 29, 2019, the Company made a line of credit loan to CannAssist, LLC (“CannAssist”), in the principal amount of up to $500,000, subject to the terms and conditions set forth in that certain Loan Agreement, dated as of January 29, 2019 between CannAssist as the Borrower and the Company as the Lender (the “CannAssist Loan Agreement”). Any draws on the line of credit in excess of $150,000 will only be made in the sole discretion of the Company. The Loan is evidenced by a secured promissory note of CannAssist (the “CannAssist Note”), which bears interest at the rate of 8% per annum and is personally guaranteed by the two equity owners of CannAssist.
To secure the obligations of CannAssist to the Company under the CannAssist Loan Agreement and the CannAssist Note, the Company and CannAssist entered into a Security Agreement dated as of January 29, 2019, pursuant to which CannAssist granted to the Company a first priority lien on and security interest in all personal property of CannAssist.
On March 11, 2019, the Company, through its wholly-owned subsidiary, CLS Massachusetts, entered into a membership interest purchase agreement (the “CannAssist Purchase Agreement”) with CannAssist, each of the members of CannAssist, and David Noble, as the members’ representative, to acquire an 80% ownership interest in CannAssist. After conducting diligence, the parties decided to terminate the CannAssist Purchase Agreement effective August 26, 2019. The CannAssist Note will be due and payable in full on or before February 28, 2020. See note 22.
On January 4, 2018, the Attorney General of the United States issued new written guidance concerning the enforcement of federal laws relating to marijuana. The Attorney General’s memorandum stated that previous DOJ guidance specific to marijuana enforcement, including the memorandum issued by former Deputy Attorney General James Cole on August 29, 2013 (as amended on February 14, 2014, the “Cole Memo”) is unnecessary and is rescinded, effective immediately. The Cole Memo told federal prosecutors that in states that had legalized marijuana, they should use their prosecutorial discretion to focus not on businesses that comply with state regulations, but on illicit enterprises that create harms like selling drugs to children, operating with criminal gangs, and selling across state lines. While the rescission did not change federal law, as the Cole Memo and other DOJ guidance documents were not themselves laws, the rescission removed the DOJ’s formal policy that state-regulated cannabis businesses in compliance with the Cole Memo guidelines should not be a prosecutorial priority. Notably, former Attorney General Sessions’ rescission of the Cole Memo has not affected the status of the U.S. Department of the Treasury’s Financial Crimes Enforcement Network (“FinCEN”) memorandum issued by the Department of Treasury, which remains in effect. This memorandum outlines Bank Secrecy Act-compliant pathways for financial institutions to service state-sanctioned cannabis businesses, which echoed the enforcement priorities outlined in the Cole Memo. In addition to his rescission of the Cole Memo, Attorney General Sessions issued a one-page memorandum known as the “Sessions Memorandum”. The Sessions Memorandum explains the DOJ’s rationale for rescinding all past DOJ cannabis enforcement guidance, claiming that Obama-era enforcement policies are “unnecessary” due to existing general enforcement guidance adopted in the 1980s, in chapter 9.27.230 of the USAM. The USAM enforcement priorities, like those of the Cole Memo, are based on the use of the federal government’s limited resources and include “law enforcement priorities set by the Attorney General,” the “seriousness” of the alleged crimes, the “deterrent effect of criminal prosecution,” and “the cumulative impact of particular crimes on the community.” Although the Sessions Memorandum emphasizes that cannabis is a federally illegal Schedule I controlled substance, it does not otherwise instruct U.S. Attorneys to consider the prosecution of cannabis-related offenses a DOJ priority, and in practice, most U.S. Attorneys have not changed their prosecutorial approach to date. However, due to the lack of specific direction in the Sessions Memorandum as to the priority federal prosecutors should ascribe to such cannabis activities, there can be no assurance that the federal government will not seek to prosecute cases involving cannabis businesses that are otherwise compliant with state law.
On October 31, 2018, the Company, CLS Massachusetts, Inc., a Massachusetts corporation and a wholly-owned subsidiary of the Company (“CLS Massachusetts”), and In Good Health, Inc., a Massachusetts corporation (“IGH”), entered into an Option Agreement (the “IGH Option Agreement”). Under the terms of the IGH Option Agreement, CLS Massachusetts has an exclusive option to acquire all of the outstanding capital stock of IGH (the “IGH Option”) during the period beginning on the earlier of the date that is one year after the effective date of the conversion and December 1, 2019 and ending on the date that is 60 days after such date. If CLS Massachusetts exercises the IGH Option, the Company, a wholly-owned subsidiary of the Company and IGH will enter into a merger agreement (the form of which has been agreed to by the parties) (the “IGH Merger Agreement”). At the effective time of the merger contemplated by the IGH Merger Agreement, CLS Massachusetts will pay a purchase price of $47,500,000, subject to reduction as provided in the IGH Merger Agreement, payable as follows: $35 million in cash, $7.5 million in the form of a five-year promissory note, and $5 million in the form of restricted common stock of the Company, plus $2.5 million as consideration for a non-competition agreement with IGH’s President, payable in the form of a five-year promissory note. IGH and certain IGH stockholders holding sufficient aggregate voting power to approve the transactions contemplated by the IGH Merger Agreement have entered into agreements pursuant to which such stockholders have, among other things, agreed to vote in favor of such transactions. On October 31, 2018, as consideration for the IGH Option, the Company made a loan to IGH, in the principal amount of $5,000,000, subject to the terms and conditions set forth in that certain loan agreement, dated as of October 31, 2018 between IGH as the borrower and the Company as the lender. The loan is evidenced by a secured promissory note of IGH, which bears interest at the rate of 6% per annum and matures on October 31, 2021. To secure the obligations of IGH to the Company under the loan agreement and the promissory note, the Company and IGH entered into a security agreement dated as of October 31, 2018, pursuant to which IGH granted to the Company a first priority lien on and security interest in all personal property of IGH. If the Company does not exercise the Option on or prior to the date that is 30 days following the end of the option period, the loan amount will be reduced to $2,500,000 as a break-up fee, subject to certain exceptions set forth in the IGH Option Agreement.
On August 26, 2019, the parties amended the IGH Option Agreement to, among other things, delay closing until January 2020.
NOTE 2 – GOING CONCERN
As shown in the accompanying financial statements, the Company has incurred net losses from operations resulting in an accumulated deficit of $46,188,151 as of May 31, 2019. Further losses are anticipated in the development of the Company’s business raising substantial doubt about the Company’s ability to continue as a going concern. The ability to continue as a going concern is dependent upon the Company generating profitable operations in the future and/or obtaining the necessary financing to meet its obligations and repay its liabilities arising from normal business operations when they come due. Management intends to finance operating costs over the next twelve months with the proceeds from the sale of securities, and/or revenues from operations. These financial statements do not include any adjustments relating to the recoverability and classification of recorded asset amounts, or amounts and classification of liabilities that might result from this uncertainty.
NOTE 3 – SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES
Basis of Presentation
These financial statements and related notes are presented in accordance with accounting principles generally accepted in the United States and are expressed in US dollars. The Company has adopted a fiscal year end of May 31st.
Principals of Consolidation
The accompanying consolidated financial statements include the accounts of CLS Holdings USA, Inc., and its wholly owned operating subsidiaries, CLS Nevada, Inc., (“CLS Nevada”), CLS Labs, Inc. (“CLS Labs”), CLS Labs Colorado, Inc. (“CLS Colorado”), CLS Massachusetts, Inc. (“CLS Massachusetts”), and Alternative Solutions, LLC (“Alternative Solutions”). Alternative Solutions is the sole owner of the following three entities (collectively, the “Oasis LLCs”): Serenity Wellness Center, LLC (“Serenity Wellness Center”); Serenity Wellness Products, LLC (“Serenity Wellness Products”); and Serenity Wellness Growers, LLC (“Serenity Wellness Growers”). All material intercompany transactions have been eliminated upon consolidation of these entities.
Use of Estimates
The preparation of financial statements in conformity with generally accepted accounting principles requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities and disclosure of contingent assets and liabilities at the date of the financial statements and the reported amount of revenues and expenses during the reporting period. Actual results could differ from those estimates.
Cash and Cash Equivalents
The Company considers all highly liquid investments with maturities of three months or less to be cash equivalents. The Company had cash and cash equivalents of $10,525,791 and $52,964 as of May 31, 2019 and 2018.
Allowance for Doubtful Accounts
The Company generates the majority of its revenues and corresponding accounts receivable from the sale of cannabis, and cannabis related products. The Company evaluates the collectability of its accounts receivable considering a combination of factors. In circumstances where it is aware of a specific customer’s inability to meet its financial obligations to it, the Company records a specific reserve for bad debts against amounts due in order to reduce the net recognized receivable to the amount it reasonably believe will be collected. For all other customers, the Company recognizes reserves for bad debts based on past write-off experience and the length of time the receivables are past due. The Company had no bad debts expense during the years ended May 31, 2019 and 2018.
Inventory
Inventories are stated at the lower of cost or market. Cost is determined on a standard cost basis that approximates the first-in, first-out (FIFO) method. Market is determined based on net realizable value. Appropriate consideration is given to obsolescence, excessive levels, deterioration, and other factors in evaluating net realizable values. Our cannabis products consist of prepackaged purchased goods ready for resale, along with produced edibles and extracts developed under our production license.
Property, Plant, and Equipment
Property and equipment is recorded at the lower of cost or estimated net recoverable amount, and is depreciated using the straight-line method over its estimated useful life. Property acquired in a business combination is recorded at estimated initial fair value. Property, plant, and equipment are depreciated using the straight-line method based on the lesser of the estimated useful lives of the assets or the lease term based upon the following life expectancy:
|
|
Years |
||
|
Office equipment |
3 to 5 |
||
|
Furniture & fixtures |
3 to 7 |
||
|
Machinery & equipment |
3 to 10 |
||
|
Leasehold improvements |
Term of lease |
||
Repairs and maintenance expenditures are charged to operations as incurred. Major improvements and replacements, which extend the useful life of an asset, are capitalized and depreciated over the remaining estimated useful life of the asset. When assets are retired or sold, the cost and related accumulated depreciation are eliminated and any resulting gain or loss is reflected in operations.
Long-Lived Assets
The Company reviews its property and equipment and any identifiable intangibles including goodwill for impairment on an annual basis utilizing the guidance set forth in the Statement of Financial Accounting Standards Board ASC 350 “Intangibles – Goodwill and Other” and ASC 360 “Property, Plant, and Equipment”. Based on Step 1 of ASC 350 and ASC 360, there were no impairments to the Company’s long-lived assets as of May 31, 2019. Long-lived assets to be disposed of are reported at the lower of carrying amount or fair value less costs to sell.
Comprehensive Income
ASC 220-10-15 “Reporting Comprehensive Income,” establishes standards for reporting and displaying of comprehensive income, its components and accumulated balances. Comprehensive income is defined to include all changes in equity except those resulting from investments by owners and distributions to owners. Among other disclosures, ASC 220-10-15 requires that all items that are required to be recognized under current accounting standards as components of comprehensive income be reported in a financial statement that is displayed with the same prominence as other financial statements. The Company does not have any items of comprehensive income in any of the periods presented.
Concentrations of Credit Risk
The Company maintains its cash in bank deposit accounts and other accounts, the balances of which at times may be uninsured or exceed federally insured limits. From time to time, some of the Company’s funds are also held by escrow agents; these funds may not be federally insured. The Company continually monitors its banking relationships and consequently has not experienced any losses in such accounts.
Advertising and Marketing Costs
All costs associated with advertising and promoting products are expensed as incurred. Total recognized advertising and marketing expenses were $1,655,374 and $0 for the years ended May 31, 2019 and 2018, respectively.
Research and Development
Research and development expenses are charged to operations as incurred. The Company incurred research and development costs of $0 for the years ended May 31, 2019 and 2018.
Fair Value of Financial Instruments
Pursuant to Accounting Standards Codification (“ASC”) No. 825 - Financial Instruments, the Company is required to estimate the fair value of all financial instruments included on its balance sheets. The carrying amounts of the Company’s cash and cash equivalents, notes receivable, convertible notes payable, accounts payable and accrued expenses, none of which is held for trading, approximate their estimated fair values due to the short-term maturities of those financial instruments.
A three-tier fair value hierarchy is used to prioritize the inputs in measuring fair value as follows:
Level 1 - Quoted prices in active markets for identical assets or liabilities.
Level 2 - Quoted prices for similar assets or liabilities in active markets, quoted prices for identical or similar assets or liabilities in markets that are not active, or other inputs that are observable, either directly or indirectly.
Level 3 - Significant unobservable inputs that cannot be corroborated by market data.
Derivative Financial Instruments
Derivatives are recorded on the condensed consolidated balance sheets at fair value. The conversion features of the convertible notes are embedded derivatives and are separately valued and accounted for on the consolidated balance sheets with changes in fair value recognized during each period of change as a separate component of other income/expense. Fair values for exchange-traded securities and derivatives are based on quoted market prices. The pricing model the Company uses for determining the fair value of its derivatives is the Lattice Model. Valuations derived from this model are subject to ongoing internal and external verification and review. The model uses market-sourced inputs such as interest rates and stock price volatilities. Selection of these inputs involves management’s judgment and may impact net income (see note 21).
On June 1, 2018, the Company adopted ASU 2017-11 and accordingly reclassified the fair value of the reset provisions embedded in convertible notes payable and certain warrants with embedded anti-dilutive provisions from liability to equity in the aggregate amount of $1,265,751.
The following assumptions were used for the valuation of the derivative liability related to the convertible notes that contain a derivative component:
For the year ended May 31, 2019
|
|
- |
That the quoted market price of the common stock, which decreased from $0.6865 as of June 1, 2018 to $0.2999 as of May 31, 2019, would fluctuate with the Company’s projected volatility; |
|
|
- |
That the conversion price of the YAN II PN Convertible Notes would be equal to $0.40 with a full reset feature, and upon default, 75% of the lowest Volume Weighted Average Price (the “VWAP”) in the 15 consecutive trading days ending on the trading day that is immediately prior to the applicable conversion date; |
|
|
- |
That the new convertible notes issued during this period with full resets would be initially issued with conversion prices of $0.40, which were not reset as a result of subsequent transactions; |
|
|
- |
That an event of default at 24% or 15% interest rate would occur 0% of the time, increasing 1.00% per month to a maximum of 25%, and that instead of a penalty, there would be an alternative conversion price; |
|
|
- |
That the projected volatility curve from an annualized analysis for each valuation period would be based on the historical volatility of the Company and the remaining term for each convertible note. The projected volatility was in the range of 97.4% to 242.8% during the year ended May 31, 2019; |
|
|
- |
That the Company would redeem the convertible notes, projected initially at 0% of the time and increasing monthly by 1.00% to a maximum of 10.0%; |
|
|
- |
That the holder would automatically convert the notes at the maximum of 2 times the conversion price or the stock price if the common stock underlying the 2017 Convertible Notes was eligible for sale in compliance with securities laws and the Company was not in default; |
|
|
- |
That unless an Event of Default occurred, the holder would sell, per trading day, an amount of Common Stock up to the greater of (i) $5,000 or (ii) 25% multiplied by the “Aggregate Amount,” as defined in the YAN II PN Convertible Notes. |
For the year ended May 31, 2018
|
|
- |
That the quoted market price of the common stock, which increased from $0.120 as of June 1, 2017 to $0.6865 as of May 31, 2018, would fluctuate with the Company’s projected volatility; |
|
|
- |
That the conversion price of the YAN II PN Convertible Notes would be equal to $0.40 with a full reset feature, and upon default, 75% of the lowest Volume Weighted Average Price (the “VWAP”) in the 15 consecutive trading days ending on the trading day that is immediately prior to the applicable conversion date; |
|
|
- |
The conversion prices of the various convertible notes would be equal to the lesser of (i) $1.07, $0.80, or $0.40 (reset to $0.03125) , as the case may be, or (ii) 75% of the lowest VWAP in the 15-20 consecutive trading days ending on the trading day that is immediately prior to the application conversion date; |
|
|
- |
That the new convertible notes issued during this period with full resets would be initially issued with conversion prices of $0.3125 and $0.40, respectively, which were not reset as a result of the WestPark Offering; |
|
|
- |
That an event of default at a 24% or 15% interest rate would occur 0% of the time, increasing 1.00% per month to a maximum of 25%, and that instead of a penalty, there would be an alternative conversion price; |
|
|
- |
That the projected volatility curve from an annualized analysis for each valuation period would be based on the historical volatility of the Company and the remaining term for each convertible note. The projected volatility was in the range of 97.4% to 534.5% during the year ended May 31, 2018; |
|
|
- |
That the Company would redeem the convertible notes, projected initially at 0% of the time and increasing monthly by 1.00% to a maximum of 10.0%; |
|
|
- |
That the holder would automatically convert the notes at the maximum of 2 times the conversion price or the stock price if the common stock underlying the YAN II PN Convertible Notes was eligible for sale in compliance with securities laws and the Company was not in default; |
|
|
- |
That unless an Event of Default occurred, the holder would sell, per trading day, an amount of Common Stock up to the greater of (i) $5,000 or (ii) 25% multiplied by the “Aggregate Amount,” as defined in the YAN II PN Convertible Notes. |
|
|
- |
That the exchange agreement conversions (contingent on the payment by Glashow to Old Main) would occur based on 95% probability; otherwise, the convertible note would revert to the original terms and settlement, and that the value of the 4,500,000 potential shares would be based on the market price as of September 25, 2017, which is the date the convertible notes were re-issued, and each conversion date price. |
Revenue Recognition
Revenue is primarily generated through the Company’s subsidiary, Serenity Wellness Center LLC, d/b/a Oasis Cannabis (“Oasis”). Oasis operates a 24-hour cannabis dispensary that recognizes revenue from the sale of medical and recreational cannabis products within the State of Nevada.
Revenue from the sale of cannabis products is recognized by our subsidiary at the point of sale, at which time payment is received. Management estimates an allowance for sales returns.
The Company also recognizes revenue from Serenity Wellness Products LLC and Serenity Wellness Growers LLC, d/b/a City Trees. City Trees recognizes revenue from the sale of the following cannabis products and services to licensed dispensaries within the State of Nevada:
|
|
● |
Premium organic medical cannabis sold wholesale to licensed retailers |
|
|
● |
Recreational marijuana cannabis products sold wholesale to distributors and retailers |
|
|
● |
Extraction products such as oils and waxes derived from in-house cannabis production |
|
|
● |
Processing and extraction services for licensed medical cannabis cultivators in Nevada |
|
|
● |
High quality cannabis strains in the form of vegetative cuttings for sale to licensed medical cannabis cultivators in Nevada |
Effective June 1, 2018, the Company adopted ASC 606 — Revenue from Contracts with Customers. Under ASC 606, the Company recognizes revenue from the commercial sales of products, licensing agreements and contracts to perform pilot studies by applying the following steps: (1) identifying the contract with a customer; (2) identifying the performance obligations in the contract; (3) determining the transaction price; (4) allocating the transaction price to each performance obligation in the contract; and (5) recognizing revenue when each performance obligation is satisfied. For the comparative periods, revenue has not been adjusted and continues to be reported under ASC 605 — Revenue Recognition. Under ASC 605, revenue is recognized when the following criteria are met: (1) persuasive evidence of an arrangement exists; (2) the performance of the service has been rendered to a customer or delivery has occurred; (3) the amount of fee to be paid by a customer is fixed and determinable; and (4) the collectability of the fee is reasonably assured.
There was no impact on the Company’s financial statements as a result of adopting Topic 606 for the year ended May 31, 2019.
Disaggregation of Revenue
The following table represents a disaggregation of revenue for the years ended May 31, 2019 and 2018:
|
2019 |
2018 |
|||||||
|
Cannabis Dispensary |
$ | 5,492,312 | $ | - | ||||
|
Cannabis Production |
2,966,736 | - | ||||||
| $ | 8,459,048 | $ | - | |||||
Basic and Diluted Earnings or Loss Per Share
Basic net earnings per share is based on the weighted average number of shares outstanding during the period, while fully diluted net earnings per share is based on the weighted average number of shares of common stock and potentially dilutive securities assumed to be outstanding during the period using the treasury stock method. Potentially dilutive securities consist of options and warrants to purchase common stock, and convertible debt. Basic and diluted net loss per share are computed based on the weighted average number of shares of common stock outstanding during the period. At May 31, 2019 and 2018, the Company excluded from the calculation of fully diluted shares outstanding the following shares because the result would have been anti-dilutive: At May 31, 2019 a total of 86,439,117 shares (54,818,985 issuable upon the exercise of warrants; 7,676,974 issuable upon exercise of unit warrants; 23,261,393 upon the conversion of convertible notes payable and accrued interest; and 681,7644 in stock to be issued); at May 31, 2018, the Company excluded from the calculation of fully diluted shares outstanding a total of 10,508,879 shares (5,521,940 issuable upon the exercise of warrants; 4,407,118 upon the conversion of notes payable and accrued interest; and 579,821 in stock payable).
The Company uses the treasury stock method to calculate the impact of outstanding stock options and warrants. Stock options and warrants for which the exercise price exceeds the average market price over the period have an anti-dilutive effect on earnings per common share and, accordingly, are excluded from the calculation.
A net loss causes all outstanding stock options and warrants to be antidilutive. As a result, the basic and dilutive losses per common share are the same for the year ended May 31, 2019 and 2018.
Income Taxes
The Company accounts for income taxes under the asset and liability method in accordance with ASC 740. The Company recognizes deferred tax liabilities and assets for the expected future tax consequences of events that have been included in the financial statements or tax returns. Under this method, deferred tax liabilities and assets are determined based on the difference between the financial statement and tax bases of assets and liabilities using enacted tax rates in effect for the year in which the differences are expected to reverse. The components of the deferred tax assets and liabilities are classified as current and non-current based on their characteristics. A valuation allowance is provided for certain deferred tax assets if it is more likely than not that the Company will not realize tax assets through future operations.
Commitments and Contingencies
Certain conditions may exist as of the date the financial statements are issued, which may result in a loss to the Company but which will only be resolved when one or more future events occur or fail to occur. The Company’s management and its legal counsel assess such contingent liabilities, and such assessment inherently involves an exercise of judgment. In assessing loss contingencies related to legal proceedings that are pending against the Company or unasserted claims that may result in such proceedings, the Company’s legal counsel evaluates the perceived merits of any legal proceedings or unasserted claims brought to such legal counsel’s attention as well as the perceived merits of the amount of relief sought or expected to be sought therein.
If the assessment of a contingency indicates that it is probable that a material loss has been incurred and the amount of the liability can be estimated, then the estimated liability would be accrued in the Company’s financial statements. If the assessment indicates that a potentially material loss contingency is not probable, but is reasonably possible, or is probable but cannot be estimated, then the nature of the contingent liability, together with an estimate of the range of possible loss if determinable and material, would be disclosed.
Loss contingencies considered remote are generally not disclosed unless they involve guarantees, in which case the nature of the guarantee would be disclosed.
Recent Accounting Pronouncements
In February 2016, the FASB issued ASU No. 2016-02, Leases (Topic 842): Accounting for Leases. This update requires that lessees recognize right-of-use assets and lease liabilities that are measured at the present value of the future lease payments at lease commencement date. The recognition, measurement, and presentation of expenses and cash flows arising from a lease by a lessee will largely remain unchanged and shall continue to depend on its classification as a finance or operating lease. The Company has performed a comprehensive review in order to determine what changes were required to support the adoption of this new standard. The Company will adopt the ASU and related amendments on June 1, 2019 and expects to elect certain practical expedients permitted under the transition guidance. The Company will elect the optional transition method that allows for a cumulative-effect adjustment in the period of adoption and will not restate prior periods. Under the new guidance, the majority of the Company’s leases will continue to be classified as operating. During the first quarter of fiscal 2020, the Company will complete its implementation of its processes and policies to support the new lease accounting and reporting requirements. Based on the Company’s lease portfolio as of June 1, 2019, the Company preliminarily estimates the impact of the adoption of ASU 2016-02 to increase both its total assets and total liabilities in the range of $850,000 to $1,050,000. The adoption of this ASU is not expected to have a significant impact on our Consolidated Statements of Operations or Cash Flows. The Company continues to finalize the implementation of the new processes and the assessment of the impact of this adoption on its consolidated financial statements; therefore, the preliminary estimated impacts disclosed can change, and the final impact will be known once the adoption is completed during the first quarter of fiscal 2020.
In May 2014, the FASB issued ASU 2014-09, Revenue from Contracts with Customers (Topic 606), to clarify the principles of recognizing revenue and create common revenue recognition guidance between U.S. GAAP and International Financial Reporting Standards. Under ASU 2014-09, revenue is recognized when a customer obtains control of promised goods or services and is recognized at an amount that reflects the consideration expected to be received in exchange for such goods or services. In addition, ASU 2014-09 requires disclosure of the nature, amount, timing, and uncertainty of revenue and cash flows arising from contracts with customers. The ASU is effective for fiscal years beginning after December 15, 2017. The new revenue standard is principle based and interpretation of those principles may vary from company to company based on their unique circumstances. It is possible that interpretation, industry practice, and guidance may evolve as companies and the accounting profession work to implement this new standard. The implementation of this standard did not have a material effect on the Company’s results of operations.
In January 2017, the FASB issued ASU No. 2017-04, Simplifying the Test for Goodwill Impairment, which simplifies the subsequent measurement of goodwill by eliminating Step 2 from the goodwill impairment test. In computing the implied fair value of goodwill under Step 2, current U.S. GAAP requires the performance of procedures to determine the fair value at the impairment testing date of assets and liabilities (including unrecognized assets and liabilities) following the procedure that would be required in determining the fair value of assets acquired and liabilities assumed in a business combination. Instead, the amendments under this ASU require the goodwill impairment test to be performed by comparing the fair value of a reporting unit with its carrying amount. An impairment charge should be recognized for the amount by which the carrying amount exceeds the reporting unit’s fair value; however, the loss recognized should not exceed the total amount of goodwill allocated to that reporting unit. The ASU becomes effective for the Company on January 1, 2020. The amendments in this ASU will be applied on a prospective basis. Early adoption is permitted for interim or annual goodwill impairment tests performed.
In August 2016, the Financial Accounting Standards Board (the “FASB”) issued ASU 2016-15, Statement of Cash Flows (Topic 230). The update addresses eight specific cash flow issues and is intended to reduce diversity in practice in how certain cash receipts and cash payments are presented and classified in the statement of cash flows. This update is effective for reporting periods beginning after December 15, 2017, including interim periods within the reporting period. Adoption of ASU 2016-15 did not have a material effect on our financial statements.
In May 2017, the FASB issued ASU No. 2017-09, Stock Compensation - Scope of Modification Accounting, which provides guidance on which changes to the terms or conditions of a share-based payment award require an entity to apply modification accounting. The ASU requires that an entity account for the effects of a modification unless the fair value (or calculated value or intrinsic value, if used), vesting conditions and classification (as equity or liability) of the modified award are all the same as for the original award immediately before the modification. The ASU became effective for the Company on January 1, 2018, and will be applied to an award modified on or after the adoption date. Adoption of ASU 2017-09 did not have a material effect on the Company’s financial statements.
Effective June 1, 2018, the Company adopted Accounting Standards Codification (“ASC”) 606 — Revenue from Contracts with Customers. Under ASC 606, the Company recognizes revenue from the commercial sales of products, licensing agreements and contracts to perform pilot studies by applying the following steps: (1) identify the contract with a customer; (2) identify the performance obligations in the contract; (3) determine the transaction price; (4) allocate the transaction price to each performance obligation in the contract; and (5) recognize revenue when each performance obligation is satisfied. For the comparative periods, revenue has not been adjusted and continues to be reported under ASC 605 — Revenue Recognition. Under ASC 605, revenue is recognized when the following criteria are met: (1) persuasive evidence of an arrangement exists; (2) the performance of service has been rendered to a customer or delivery has occurred; (3) the amount of fee to be paid by a customer is fixed and determinable; and (4) the collectability of the fee is reasonably assured. There was no impact on the Company’s financial statements as a result of adopting ASC 606.
On June 1, 2018, the Company adopted ASU 2017-11 and accordingly reclassified the fair value of the reset provisions embedded in convertible notes payable and certain warrants with embedded anti-dilutive provisions from liability to equity in the aggregate amount of $1,265,751.
There are various other updates recently issued, most of which represented technical corrections to the accounting literature or application to specific industries and are not expected to a have a material impact on the Company’s consolidated financial position, results of operations or cash flows.
NOTE 4 – ACQUISITION OF ALTERNATIVE SOLUTIONS
On June 27, 2018, the Company closed on the purchase of all of the membership interests in Alternative Solutions and its three operating subsidiaries (collectively, the “Oasis LLCs”) from the members of such entities (other than Alternative Solutions). The Oasis LLCs operate a fully integrated cannabis business in Las Vegas, Nevada, including a grow; extraction, conversion and processing facility; and a retail dispensary. The closing occurred pursuant to a Membership Interest Purchase Agreement (the “Acquisition Agreement”) entered into between the Company and Alternative Solutions on December 4, 2017, as amended. Pursuant to the Acquisition Agreement, the Company initially contemplated acquiring all of the membership interests in the Oasis LLCs from Alternative Solutions. Just prior to closing, the parties agreed that the Company would instead acquire all of the membership interests in Alternative Solutions, the parent of the Oasis LLCs, from its members, and the membership interests in the Oasis LLCs owned by members other than Alternative Solutions. The revised structure of the transaction is referenced in the Oasis Note, which modified the Acquisition Agreement.
Pursuant to the Acquisition Agreement, the Company paid a non-refundable deposit of $250,000 upon signing, which was followed by an additional payment of $1,800,000 paid in February 2018, for an initial 10% of each of the Oasis LLCs. At that time, the Company applied for regulatory approval to own an interest in the Oasis LLCs, which approval was received. On June 27, 2018, the Company made the payments to indirectly acquire the remaining 90% of the Oasis LLCs, which were equal to cash in the amount of $5,995,543, a $4.0 million promissory note due in December 2019 (see note 14), (the “Oasis Note”), and 22,058,823 shares of its common stock (see note 16), (the “Purchase Price Shares”) (collectively, the “Closing Consideration”). The cash payment of $5,995,543 was less than the $6,200,000 payment originally contemplated because the Company assumed an additional $204,457 of liabilities. The Company used the proceeds of a Canadian private securities offering to fund the cash portion of the Closing Consideration (see note 16). The Company then applied for regulatory approval to own the additional 90% in membership interests in the Oasis LLCs, which it received on December 12, 2018.
The number of Purchase Price Shares was equal to 80% of the offering price of the Company’s common stock in its last equity offering, which price was $0.34 per share. The Oasis Note is secured by a first priority security interest over the membership interests in Alternative Solutions and the Oasis LLCs, as well as by the assets of the Oasis LLCs. The Company also delivered a confession of judgment to a representative of the sellers that will become effective, in general, if the Company defaults under the Oasis Note.
A claim has been made that Oasis owes certain amounts to a consultant; Oasis disputes this claim. If the Company makes any payments in connection with this claim post-closing, generally speaking, the Company will be entitled to deduct the present value of such payments from the principal amount due under the Oasis Note. This claim has been accrued on the Company’s balance sheet as of May 31, 2019. This claim was resolved subsequent to May 31, 2019 (see note 22).
The sellers are also entitled to a $1,000,000 payment from the Company on May 30, 2020 if the Oasis LLCs have maintained an average revenue of $20,000 per day during the 2019 calendar year. The fair value of this contingent consideration was $678,111 at the acquisition date as determined by the Company’s outside valuation consultants. At May 31, 2019, the Company increased the value of this contingent consideration to $1,000,000, and charged the amount of $321,889 to operations during the year ended May 31, 2019. This amount is recorded as a contingent liability on the Company’s balance sheet at May 31, 2019.
The acquisition date estimated fair value of the consideration transferred totaled $27,975,650, which consisted of the following:
|
Initial purchase price |
$ | 2,050,000 | ||
|
Cash paid in connection with transaction |
5,995,543 | |||
|
Note payable |
3,810,820 | |||
|
Contingent consideration |
678,111 | |||
|
Common stock |
15,441,176 | |||
|
Total purchase price |
$ | 27,975,650 | ||
|
Net tangible assets |
$ | 595,151 | ||
|
Intangible assets |
1,637,600 | |||
|
Goodwill |
25,742,899 | |||
|
Total purchase price |
$ | 27,975,650 |
The above estimated fair value of the intangible assets is based on a preliminary purchase price allocation prepared by a third party valuation expert. During the preliminary purchase price allocation period, which may be up to one year from the business combination date, the Company may record adjustments to the assets acquired and liabilities assumed, with the corresponding offset to goodwill. After the preliminary purchase price allocation period, the Company may record adjustments to assets acquired or liabilities assumed subsequent to the purchase price allocation period in its operating results in the period in which the adjustments were determined.
Pro forma results
The following table sets forth the unaudited pro forma results of the Company as if the acquisition of the Oasis LLCs was effective on the first day of each of the twelve months periods presented. These combined results are not necessarily indicative of the results that may have been achieved had the companies always been combined.
|
Twelve months ended May 31, |
||||||||
|
2019 |
2018 |
|||||||
|
(unaudited) |
(unaudited) |
|||||||
|
Revenues |
$ | 9,759,956 | $ | 7,258,443 | ||||
|
Net loss |
$ | (26,671,841 |
) |
$ | (18,885,612 |
) |
||
|
Basic net loss per share |
$ | (0.26 |
) |
$ | (0.30 |
) |
||
|
Diluted net loss per share |
$ | (0.26 |
) |
$ | (0.30 |
) |
||
|
Weighted average shares - basic |
102,869,612 | 62,558,436 | ||||||
|
Weighted average shares - diluted |
102,869,612 | 62,558,436 | ||||||
NOTE 5 – JOINT VENTURE AND OPTIONS TRANSACTION
In Good Health
On October 31, 2018, the Company, CLS Massachusetts, Inc., a Massachusetts corporation and a wholly-owned subsidiary of the Company (“CLS Massachusetts”), and In Good Health, Inc. (“IGH”), a Massachusetts not-for-profit corporation, which converted to a for-profit corporation on November 6, 2018 (the “Conversion”), entered into an Option Agreement (the “Option Agreement”). Under the terms of the Option Agreement, CLS Massachusetts has an exclusive option to acquire all of the outstanding capital stock of IGH (the “Option”) during the period beginning on the earlier of the date that is one year after the effective date of the Conversion and December 1, 2019, and ending on the date that is 60 days after such date (the “Option Period”). If CLS Massachusetts exercises the Option, the Company, a wholly-owned subsidiary of the Company and IGH will enter into a merger agreement (the form of which has been agreed to by the parties) (the “IGH Merger Agreement”). At the effective time of the merger contemplated by the IGH Merger Agreement, CLS Massachusetts will pay a purchase price of $47,500,000, subject to reduction as provided in the IGH Merger Agreement, payable as follows: $35 million in cash, $7.5 million in the form of a five-year promissory note, and $5 million in the form of restricted common stock of the Company, plus $2.5 million as consideration for a non-competition agreement with IGH’s President, payable in the form of a five-year promissory note.
IGH and certain IGH stockholders holding sufficient aggregate voting power to approve the transactions contemplated by the IGH Merger Agreement have entered into agreements pursuant to which such stockholders have, among other things, agreed to vote in favor of such transactions.
On October 31, 2018, as consideration for the Option, the Company made a loan to IGH (the “IGH Loan”), in the principal amount of $5,000,000 (the “IGH Loan Amount”), subject to the terms and conditions set forth in that certain Loan Agreement, dated as of October 31, 2018 between IGH as the borrower and the Company as the lender (the “IGH Loan Agreement”) (see note 9). The IGH Loan is evidenced by a secured promissory note of IGH (the “IGH Note”), which bears interest at the rate of 6% per annum and matures on October 31, 2021. The Company recorded interest income in the amount of $174,247 on the IGH Loan during the year ended May 31, 2019.
To secure the obligations of IGH to the Company under the Loan Agreement and the IGH Note, the Company and IGH entered into a Security Agreement dated as of October 31, 2018 (the “IGH Security Agreement”), pursuant to which IGH granted to the Company a first priority lien on and security interest in all personal property of IGH.
If the Company does not exercise the Option on or prior to the date that is 30 days following the end of the Option Period, the Loan Amount will be reduced to $2,500,000 as a break-up fee (the “Break-Up Fee”), except in the event of a Purchase Exception (as defined in the Option Agreement), in which case the Break-Up Fee will not apply and there will be no reduction to the Loan Amount.
CannAssist
On January 29, 2019, the Company made a line of credit loan to CannAssist in the principal amount of up to $500,000, subject to the terms and conditions set forth in the CannAssist Loan Agreement. Any draws on the line of credit in excess of $150,000 will only be made in the sole discretion of the Company. The Loan is evidenced by the CannAssist Note, which bears interest at the rate of 8% per annum and is personally guaranteed by the two equity owners of CannAssist. The Company recorded interest income in the amount of $4,011 on the Loan during the year ended May 31, 2019.
To secure the obligations of CannAssist to the Company under the CannAssist Loan Agreement and the CannAssist Note, the Company and CannAssist entered into a Security Agreement dated as of January 29, 2019, pursuant to which CannAssist granted to the Company a first priority lien on and security interest in all personal property of CannAssist.
On March 11, 2019, the Company, through its wholly-owned subsidiary, CLS Massachusetts, entered into the CannAssist Purchase Agreement with CannAssist, each of the members of CannAssist, and David Noble, as the members’ representative.
NOTE 6 – ACCOUNTS RECEIVABLE
Accounts receivable was $163,571 and $0 at May 31, 2019 and 2018, respectively. No allowance for doubtful accounts was necessary during the years ended May 31, 2019 and 2018.
NOTE 7 – PREPAID EXPENSES AND OTHER CURRENT ASSETS
Prepaid expenses and other current assets consisted of the following at May 31, 2019 and 2018:
|
May 31, |
May 31, |
|||||||
|
2019 |
2018 |
|||||||
|
Deposits |
$ | 211,493 | - | |||||
|
Prepaid expenses |
178,920 | 1,410 | ||||||
|
Total |
$ | 390,413 | $ | 1,410 | ||||
NOTE 8 – INVENTORY
Inventory, consisting of material, overhead, labor, and manufacturing overhead, is stated at the lower of cost (first-in, first-out) or market, and consists of the following:
|
May 31, |
May 31, |
|||||||
|
2019 |
2018 |
|||||||
|
Raw materials |
$ | 323,635 | $ | - | ||||
|
Finished goods |
423,198 | - | ||||||
|
Total |
$ | 746,833 | $ | - | ||||
Raw materials consist of cannabis plants and the materials that are used in our production process prior to being tested and packaged for consumption. Finished goods consist of pre-packaged materials previously purchased from other licensed cultivators and our manufactured edibles and extracts.
NOTE 9 – NOTES RECEIVABLE
PRH Note Receivable
During the year ended May 31, 2015, the Company loaned $500,000 (the “PRH Note”) to Picture Rock Holdings, LLC, a Colorado limited liability company (“PRH”). Pursuant to the PRH Note, as amended by the parties effective June 30, 2015, October 31, 2015, April 11, 2016, and May 31, 2016, PRH was expected to repay the principal due under the PRH Note in twenty (20) equal quarterly installments of Twenty Five Thousand Dollars ($25,000) commencing in the month following the month in which PRH commenced generating revenue at the grow facility, which commencement was originally anticipated to occur in the first quarter of 2017, and continuing until paid in full. We suspended our plans to operate in Colorado due to regulatory delays and have not yet determined when we will pursue them again. Interest will accrue on the unpaid principal balance of the PRH Note at the rate of twelve percent (12%) per annum and will be paid quarterly in arrears commencing after such initial payment and continuing until paid in full. All outstanding principal and any accumulated unpaid interest due under the PRH Note is due and payable on the five-year anniversary of the initial payment thereunder. In the event of default as defined in the agreements underlying the PRH Note, all amounts under the PRH Note shall be due and payable at once. During the year ended May 31, 2015, the Company recorded an impairment related to the note receivable in the amount of $500,000.
During the year ended May 31, 2018, the Company received a payment of $50,000 on the PRH Note. As a result, the Company has reduced the impairment of the note by $50,000 to reflect this payment. The receivable is recorded on the balance sheet as of May 31, 2019 in the amount of $0, net of allowance in the amount of $450,000.
IGH Note Receivable
On October 31, 2018, in connection with an option to purchase transaction (see note 4), the Company loaned $5,000,000 (the “IGH Note) to In Good Health, Inc., a Massachusetts not-for-profit corporation (“IGH”); on November 6, 2018, IGH converted to a for-profit corporation. The IGH Note bears interest at the rate of 6% per annum. On March 1, 2020 (the “Initial Payment Date”), all accrued interest shall be added to the outstanding principal due hereunder and such amount shall be payable in eight equal quarterly installments, commencing on the Initial Payment Date, together with interest accruing after the Initial Payment Date. The IGH Note shall mature and all outstanding principal, accrued interest and any other amounts due hereunder, shall become due and payable in full on the third anniversary of the IGH Note. The IGH Note was issued in connection with a loan agreement and security agreement between the Company and IGH, and an option agreement between the Company and IGH, among others (the “Option Agreement”), in both cases dated as of October 31, 2018 and the other agreements and documents executed and/or delivered in connection therewith (collectively the “IGH Loan Documents”), and is secured by the collateral described in the IGH Loan Documents and by such other collateral as may in the future be granted to the Company to secure the IGH Note. During the year ended May 31, 2019, the Company recorded interest income in the amount of $174,247 in connection with the IGH Note. At May 31, 2019, principal in the amount of $425,479 and interest receivable in the amount of $174,247 due under the IGH Note are classified as current assets and principal in the amount of $4,724,521 is classified as non-current assets on the Company’s balance sheet.
CannAssist Note Receivable
On January 29, 2019, the Company made a line of credit loan to CannAssist (the “CannAssist Note”), in the principal amount of up to $500,000. The Loan bears interest at the rate of 8% per annum and is personally guaranteed by the two equity owners of CannAssist. Payments on the loan will commence on July 1, 2019 and the Note will mature on December 1, 2019. During the year ended May 31, 2019, the Company recorded interest income in the amount of $4,011 on the CannAssist Note. At May 31, 2019, the principal amount of $150,000 and interest receivable in the amount of $4,011 are due under the CannAssist Note and are classified as current assets on the Company’s balance sheet.
NOTE 10 – PROPERTY, PLANT AND EQUIPMENT
Property, plant and equipment consisted of the following at May 31, 2019 and 2018.
|
May 31, |
May 31, |
|||||||
|
2019 |
2018 |
|||||||
|
Office equipment |
$ | 53,152 | $ | 2,674 | ||||
|
Furniture & fixtures |
140,701 | - | ||||||
|
Machinery & equipment |
969,196 | - | ||||||
|
Leasehold improvements |
1,293,660 | - | ||||||
|
Less: accumulated depreciation |
(546,408 |
) |
(2,674 |
) |
||||
|
Property and equipment, net |
$ | 1,910,301 | $ | - | ||||
During the year ended May 31, 2019, the Company acquired property, plant, and equipment with an aggregate fair value of $933,142 with the acquisition of Alternative Solutions. See note 4. The Company also made payments in the amount of $1,037,262 for property and equipment during the year ended May 31, 2019.
Depreciation expense totaled $173,277 and $890 for the years ended May 31, 2019 and 2018, respectively.
NOTE 11 – INTANGIBLE ASSETS
Intangible assets consisted of the following at May 31, 2019 and 2018:
|
May 31, 2019 |
Accumulated |
|||||||||||
|
Gross |
Amortization |
Net |
||||||||||
|
Intellectual Property |
$ | 319,600 | $ | (29,297 |
) |
$ | 290,303 | |||||
|
License & Customer Relations |
990,000 | (45,375 |
) |
944,625 | ||||||||
|
Tradenames - Trademarks |
301,000 | (27,592 |
) |
273,408 | ||||||||
|
Non-compete Agreements |
27,000 | (12,378 |
) |
14,622 | ||||||||
|
Domain Names |
3,963 | (1,834 |
) |
2,129 | ||||||||
|
Total |
$ | 1,641,563 | $ | (116,476 |
) |
$ | 1,525,087 | |||||
|
May 31, 2018 |
Accumulated |
|||||||||||
|
Gross |
Amortization |
Net |
||||||||||
|
Intellectual Property |
$ | - | $ | - | $ | - | ||||||
|
License & Customer Relations |
- | - | - | |||||||||
|
Tradenames - Trademarks |
- | - | - | |||||||||
|
Non-compete Agreements |
- | - | - | |||||||||
|
Domain names |
2,300 | (1,402 |
) |
898 | ||||||||
|
Total |
$ | 2,300 | $ | (1,402 |
) |
$ | 898 | |||||
Total amortization expense charged to operations for the years ended May 31, 2019 and 2018 was $115,074 and $432, respectively.
|
Amount to be amortized during the twelve months ended May 31, |
||||
|
2020 |
$ | 125,600 | ||
|
2021 |
114,271 | |||
|
2022 |
111,560 | |||
|
2023 |
111,560 | |||
|
2024 |
111,560 | |||
|
Thereafter |
950,536 | |||
| $ | 1,525,087 |
NOTE 12 – GOODWILL
The Company recorded goodwill in the amount of $25,742,899 in connection with the acquisition of Alternative Solutions on June 27, 2018 (see note 4). Goodwill is tested for impairment on an annual basis utilizing the two-step process set forth in ASC 350 and ASC 360. The first step of this process compares the book value of the Company with its fair value. If the fair value exceeds its carrying amount (including recorded goodwill), no goodwill impairment has occurred. Since the fair value of the Company based upon market price of the Company’s common stock exceeded the carrying value (including goodwill) at May 31, 2019, no indication of goodwill impairment exists.
NOTE 13 – OTHER ASSETS
Other assets included the following as of May 31, 2019 and May 31, 2018:
|
February 28, |
May 31, |
|||||||
|
2019 |
2018 |
|||||||
|
Security deposits |
167,455 | - | ||||||
| $ | 167,455 | $ | - | |||||
NOTE 14 – ACCOUNTS PAYABLE AND ACCRUED LIABILITIES
Accounts payable and accrued liabilities consisted of the following at May 31, 2019 and 2018:
|
May 31, |
May 31, |
|||||||
|
2019 |
2018 |
|||||||
|
Trade accounts payable |
$ | 510,210 | $ | 726,457 | ||||
|
Accrued payroll and payroll taxes |
230,119 | 44,465 | ||||||
|
Accrued liabilities |
625,399 | - | ||||||
|
Deferred rent liability |
151,399 | 55,699 | ||||||
|
Total |
$ | 1,517,127 | $ | 826,621 | ||||
NOTE 15 – NOTES PAYABLE AND CONVERTIBLE NOTES PAYABLE
Notes Payable
|
May 31, |
May 31, |
|||||||
|
2019 |
2018 |
|||||||
|
On February 7, 2018, the Company issued a note payable to Todd Blatt in the amount of $210,000 the “Blatt Note”). This note accrues interest at a rate of 6% per annum and is due on February 7, 2019. During the year ended May 31, 2019, the Company accrued interest in the amount of $1,726 on the Blatt Note. On July 20, 2018, the Company made principal and interest payments in the amount of $210,000 and $5,627, respectively, on the Blatt Note. |
$ | - | $ | 210,000 | ||||
|
On February 7, 2018, the Company issued a note payable to AJG Group in the amount of $200,000 the “AJG Note”). This note accrues interest at a rate of 6% per annum and is due on February 7, 2019. During the year ended May 31, 2018, the Company made a principal payment in the amount of $100,000 and accrued interest in the amount of $2,696 on the AJG note. During the year ended May 31, 2019, the Company accrued interest in the amount of $641 on the AJG Note. On July 9, 2018, the Company made principal and interest payments in the amount of $100,000 and $3,337, respectively, on the AJG Note. |
- | 100,000 | ||||||
|
The Company issued a secured note payable to Serenity Wellness Enterprises, LLC, as nominee (“Oasis Note”). dated June 27, 2018 in the principal amount of $4,000,000 and bearing interest at a rate of 6% per annum pursuant to the Membership Interest Purchase Agreement with Alternative Solutions. The note is due on December 4, 2019, but may be prepaid at any time without penalty. The Oasis Note is secured by all of the membership interests in Alternative Solutions and the Oasis LLCs and by the assets of the Oasis LLCs.
The Company recognized an original issue discount of $189,180 on the Oasis Note. During the year ended May 31, 2019, $121,796 of this discount was charged to operations. During the year ended May 31, 2019, the Company accrued interest in the amount of $225,333 on the Oasis Note. |
4,000,000 | - | ||||||
|
Total – Notes Payable |
$ | 4,000,000 | $ | 310,000 | ||||
|
Less: Discount |
(67,384 |
) |
- | |||||
|
Notes Payable, Net of Discounts |
$ | 3,932,616 | $ | 310,000 | ||||
|
Current portion |
$ | 3,932,616 | $ | 310,000 | ||||
|
Long term portion |
$ | - | $ | - | ||||
Related Party Convertible Demand Notes Payable
On May 31, 2017, the Company entered into an Omnibus Loan Amendment Agreement (the “Omnibus Loan Amendment”) with Jeffrey I. Binder, Frank Koretsky, Newcan Investment Partners LLC and CLS CO 2016, LLC (collectively, the “Insiders”). Pursuant to the Omnibus Loan Amendment, the Company agreed with the Insiders to amend certain terms of loans the Insiders made to the Company for working capital purposes, which loans were initially demand loans, and, except for loans made in 2017, were later memorialized as convertible loans (the “Insider Loans”), in exchange for the agreement of the Insiders to convert all Insider Loans where funds were advanced prior to January 1, 2017, which totaled $2,537,750, plus $166,490 of accrued interest thereon, into an aggregate of 10,816,960 shares of the Company’s common stock at $0.25 per share, and forego the issuance of warrants to purchase the Company’s common stock upon conversion. This resulted in the issuance of an additional 7,609,910 shares compared to the original number of shares issuable upon conversion of the Insider Loans prior to the Omnibus Loan Amendment. The Company valued the shares at $0.125, which was the market price of the Company’s stock at the conversion date, and charged the amount of $951,239 to loss on modification of debt during the year ended May 31, 2017. The Company entered into the Omnibus Loan Amendment in order to ease the debt burden on the Company and prevent it from defaulting on the Insider Loans.
Pursuant to the Omnibus Loan Amendment, the following amendments were made to the Insider Loans: (a) the Company reduced the conversion price on the Insider Loans from between $0.75 and $1.07 per share of common stock to $0.25 per share of common stock, in those cases where the conversion price was greater than $0.25, which reduced conversion price exceeded the closing price of the common stock during the three months prior to the Omnibus Loan Amendment; (b) the Company deleted the requirement to issue warrants to purchase the Company’s common stock upon conversion of the Insider Loans; (c) the Company amended one Insider Loan to permit conversion of only the portion of the Insider Loan related to services that were provided to it prior to January 1, 2017; and (d) the Company amended the terms of the Insider Loans where funds were advanced on or after January 1, 2017, which Insider Loans were not converted into the Company’s common stock, to provide for, where not already the case, a 10% interest rate per annum, a $0.25 conversion price per share of common stock, and the deletion of the requirement that the Company issue warrants to purchase its common stock upon conversion of such Insider Loans.
On January 10, 2018, effective December 1, 2017, the Company entered into an Omnibus Amendment to Convertible Notes (the “Second Omnibus Loan Agreement”) with Jeffrey I. Binder, an officer and director of the Company, and Newcan Investment Partners LLC, an entity owned by Frank Koretsky, a director of the Company. The Second Omnibus Loan Agreement provides that the conversion price of all outstanding convertible promissory notes issued to either Mr. Binder or Newcan Investment Partners, LLC as of the date of the Agreement would be increased from $0.25 to $0.3125 per share of common stock. The remaining terms of such notes remain unchanged.
The following tables summarize the Company’s loan balances at May 31, 2019 and 2018:
|
May 31, |
May 31, |
|||||||
|
2019 |
2018 |
|||||||
|
Notes payable to Jeffrey Binder, an officer and director of the Company, for advances to fund operations (the “Binder Funding Notes”). The Binder Funding Notes bear interest at a rate of 10% per annum, have no maturity date and are due on demand. Effective May 31, 2017, pursuant to the Omnibus Loan Amendment, a conversion feature was added to the Binder Funding Notes whereby principal and accrued interest is convertible into common stock of the Company at a rate of $0.25 per share.
Effective December 1, 2017, pursuant to the Second Omnibus Loan Amendment, the conversion price was increased from $0.25 per share to $0.3125 per share and a discount in the amount of $35,023 related to the revaluation of the beneficial conversion feature of the Binder Funding Notes was charged to additional paid-in capital and amortized to interest expense.
During the year ended May 31, 2018, Mr. Binder advanced a total of $440,579 to the Company under the Binder Funding Notes. During the year ended May 31, 2018, principal in the amount of $280,198 and accrued interest in the amount of $5,188 was transferred out of the Binder Funding Notes and used to fund four new convertible notes payable to Mr. Binder, which were converted or repaid as of May 31, 2018. Also during the year ended May 31, 2018, the Company made principal payments in the aggregate of $237,794 under the Binder Funding Notes. During the year ended May 31, 2018, the Company accrued interest in the amount of $7,364 on the Binder Funding Notes. During the year ended May 31, 2018, discounts in the amount of $385,637 related to the beneficial conversion feature of the Binder Funding Notes was charged to additional paid-in capital and amortized to interest expense.
During the year ended May 31, 2019, the Company paid principal and accrued interest in the amounts of $137 and $3,338, respectively, on the Binder Funding Notes. |
$ | - | $ | 137 | ||||
|
May 31, 2019 |
May 31, 2018 |
|||||||
|
Notes payable to Newcan Investment Partners, LLC (“Newcan”), an entity owned by Frank Koretsky, a director of the Company, for advances to fund operations (the “Newcan Funding Notes”). The Newcan Funding Notes bear interest at a rate of 10% per annum, have no maturity date and are due on demand. Effective May 31, 2017, pursuant to the Omnibus Loan Agreement, a conversion feature was added to the Newcan Funding Notes whereby principal and accrued interest is convertible into common stock of the Company at a rate of $0.25 per share.
Effective December 1, 2017, pursuant to the Second Omnibus Loan Amendment, the conversion price was increased from $0.25 per share to $0.3125 per share and a discount in the amount of $6,120 related to the revaluation of the beneficial conversion feature of the Newcan Funding Notes was charged to additional paid-in capital and amortized to interest expense.
During the year ended May 31, 2018, Newcan advanced a total of $290,000 to the Company under the Newcan Funding Notes. During the year ended May 31, 2018, principal in the amount of $836,658 and accrued interest in the amount of $25,018 was transferred out of the Newcan Funding Notes and used to fund four new convertible notes payable to Newcan, which were converted or repaid as of May 31, 2018. During the year ended May 31, 2018, the Company accrued interest in the amount of $16,681 on the Newcan Funding Notes. During the year ended May 31, 2018, discounts in the amount of $210,120 related to the beneficial conversion feature of the Newcan Funding Notes were charged to additional paid-in capital and amortized to interest expense.
During the year ended May 31, 2019, principal in the amount of $75,000 and accrued interest in the amount of $1,931 was transferred out of the Newcan Funding Notes and used to create a new convertible note payable to Newcan (“Newcan Convertible Note 8”). During the year ended May 31, 2019, the Company accrued interest in the amount of $1,377 on the Newcan Funding Notes. |
- | 75,000 | ||||||
|
Total – Demand Convertible Notes Payable, Related Parties |
$ | - | $ | 75,137 | ||||
|
Total – Demand Convertible Notes Payable, Related Parties - Current portion |
$ | - | $ | 75,137 | ||||
|
Total – Demand Convertible Notes Payable, Related Parties - Long term portion |
$ | - | $ | - | ||||
Convertible Notes Payable, Related Parties
|
May 31, 2019 |
May 31, 2018 |
|||||||
|
Convertible promissory note payable to David Lamadrid (the “Lamadrid Note”) dated February 20, 2018 in the principal amount of $31,250 and bearing interest at a rate of 8% per annum. The Lamadrid Note is due eighteen months from the date of issue. Mr. Lamadrid may, at his option, convert all or a portion of the Lamadrid Note and accrued but unpaid interest into shares of common stock at a conversion price of $0.3125 per share. The Lamadrid Note also contains a reset feature, whereby, absent certain exceptions, if the Company issues equity securities at an effective price less than $0.3125 per share of common stock, the conversion price of the Lamadrid Note will be reset to such lower price. The Company recognized a discount of $31,250 on the Lamadrid Note related to the beneficial conversion feature at the time of issuance. During the year ended May 31, 2018, $942 of this discount was charged to operations. During the year ended May 31, 2018, the Company accrued interest in the amount of $685 on this note.
During the year ended May 31, 2019, interest in the amount of $562 was accrued on the Lamadrid note. During the year ended May 31, 2019, the Lamadrid Note, in the amount of $32,497, of which $31,250 was principal and $1,247 was accrued interest, was converted into 103,989 shares of common stock. During the year ended May 31, 2019, the remaining discount in the amount of $30,308 was charged to operations. |
$ | - | $ | 31,250 | ||||
|
Unsecured convertible note issued to Jeffery Binder, an officer and director of the Company, dated April 6, 2018 in the original principal amount of $37,500 (the “Binder Convertible Note 9”). The Binder Convertible Note 9 was funded with the conversion of $37,500 of unpaid accrued salary due to Mr. Binder. This note bears interest at the rate of 10% per annum. No interest payments are required until July 1, 2019, at which time all accrued interest becomes due and payable. Commencing October 1, 2019, the first of eight principal payments in the amount of $4,688 will become due; subsequent payments will become due on the first day of each January, April, July and October until paid in full. This note and accrued interest under the note may be converted, in whole or in part, into one share of common stock for each $0.3125 converted. The Company recognized a discount of $37,500 on the Binder Convertible Note 9 related to the value of the beneficial conversion feature at the time of issuance. During the year ended May 31, 2018, the Company amortized $1,890 of this discount to interest expense.
During the year ended May 31, 2019, interest in the amount of $699 was accrued on the Binder Convertible Note 9. During the year ended May 31, 2019, the Company made principal and interest payments in the amount of $37,500 and $1,264, respectively, on the Binder Convertible Note 9. During the year ended May 31, 2019 the remaining discount in the amount of $35,610 was charged to operations. |
- | 37,500 | ||||||
|
May 31, 2019 |
May 31, 2018 |
|||||||
|
Unsecured convertible note issued to Newcan, an entity owned by Frank Koretsky, a director of the Company, dated, August 6, 2018 in the original amount of $75,000 (the “Newcan Convertible Note 8”). The Newcan Convertible Note 8 was funded with the conversion of $75,000 of advances Newcan made to the Company under the Newcan Funding Notes. This note bears interest at the rate of 10% per annum. No interest payments are required until October 1, 2019, at which time all of the accrued interest becomes due and payable. Commencing on January 1, 2020, the first of eight principal payments in the amount of $9,375 will become due; subsequent principal payments will become due on the first day of each April, July, October, and January until paid in full. This note and accrued interest under the note may be converted, in whole or in part, into one share of common stock for each $0.40 converted. The Company recognized a discount of $58,594 on the Newcan Convertible Note 8 related to the value of the beneficial conversion feature at the time of issuance.
During the year ended May 31, 2019, the Company accrued interest expense in the amount of $1,603 on the Newcan Convertible Note 8. During the year ended May 31, 2019, the note holder converted $78,534, of which $75,000 was principal and $3,534 was accrued interest into 196,336 shares of common stock. Also during the year ended May 31, 2019, the remaining discount in the amount of $57,322 was charged to operations. |
- | - | ||||||
|
Total – Convertible Notes Payable, Related Parties |
$ | - | $ | 68,750 | ||||
|
Less: Discount |
- | (65,918 |
) |
|||||
|
Convertible Notes Payable, Related Parties, Net of Discounts |
$ | - | $ | 2,832 | ||||
|
Convertible Notes Payable, Related Parties, Net of Discounts, Current Portion |
$ | - | $ | 2,832 | ||||
|
Convertible Notes Payable, Related Parties, Net of Discounts, Long-term Portion |
- | - | ||||||
Convertible Notes Payable
|
May 31, 2019 |
May 31, 2018 |
|||||||
|
Convertible promissory note payable to Darling Capital, LLC (the “Darling Note”) dated February 5, 2018 and bearing interest at a rate of 8% per annum. The lender loaned the Company $500,000 and the Darling Note has an original issue discount of $50,000. The Darling Note is due eighteen months from the date of issue. Darling may, at its option, convert all or a portion of the Darling Note and accrued but unpaid interest into shares of common stock at a conversion price of $0.3125 per share. The Darling Note also contains a reset feature, whereby, absent certain exceptions, if the Company issues equity securities at an effective price less than $0.3125 per share of common stock, the conversion price of the Darling Note will be reset to such lower price. The Company recognized a discount of $550,000 on the Darling Note related to the beneficial conversion feature at the time of issuance. During the year ended May 31, 2018, $40,427 of this discount was charged to operations. During the year ended May 31, 2018, the Company accrued interest in the amount of $13,863 on this note.
During the year ended May 31, 2019, the Company accrued interest in the amount of $1,447 on this note. During the year ended May 31, 2019, the holder of the Darling Note converted $565,000, of which $550,000 was principal and $15,000 was accrued interest, into 1,808,000 shares of common stock. Also, during the year ended May 31, 2019, the remaining discount in the amount of $509,573 was charged to operations. |
$ | - | $ | 550,000 | ||||
|
Convertible promissory note payable to Efrat Investments, LLC (the “Efrat Note”) dated February 12, 2018 and bearing interest at a rate of 8% per annum. The lender loaned the Company $50,000 and the Efrat Note has an original issue discount of $5,000. The Efrat Note is due eighteen months from the date of issue. Efrat may, at its option, convert all or a portion of the Efrat Note and accrued but unpaid interest into shares of common stock at a conversion price of $0.3125 per share. The Efrat Note also contains a reset feature, whereby, absent certain exceptions, if the Company issues equity securities at an effective price less than $0.3125 per share of common stock, the conversion price of the Efrat Note will be reset to such lower price. The Company recognized a discount of $55,000 on the Efrat Note related to the beneficial conversion feature at the time of issuance. During the year ended May 31, 2018, $2,974 of this discount was charged to operations. During the year ended May 31, 2018, the Company accrued interest in the amount of $1,302 on this note.
During the year ended May 31, 2019, the Company accrued interest in the amount of $898 on this note. During the year ended May 31, 2019, the holder of the Efrat Note converted $57,200, of which $55,000 was principal and $2,200 was accrued interest into 183,040 shares of common stock. Also during the year ended May 31, 2019, the remaining discount in the amount of $52,026 was charged to operations. |
- | 55,000 | ||||||
|
May 31, 2019 |
May 31, 2018 |
|||||||
|
Convertible promissory note payable to YA II PN, Ltd. (the “YA II PN Note”) dated May 14, 2018 and bearing interest at a rate of 8% per annum. The lender loaned the Company $750,000, and the note is due on November 14, 2019. YA II PN may, at its option, convert all or a portion of the YA II PN Note and accrued but unpaid interest into shares of common stock at a conversion price of $0.40 per share. The YA II PN Note also contains a reset feature, whereby, absent certain exceptions, if the Company issues equity securities at an effective price less than $0.40 per share of common stock, the conversion price of the YA II PN Note will be reset to such lower price. The Company recognized a discount of $750,000 related to the beneficial conversion feature at the time of issuance. Commencing on December 1, 2018, absent certain exceptions, the first of eight payments in the amount of $93,750 will become due; subsequent payments will become due on the first day of each of the following months until paid in full. During the year ended May 31, 2018, $23,224 of this discount was charged to operations. During the year ended May 31, 2018, the Company accrued interest in the amount of $2,795 on this note.
During the year ended May 31, 2019, a reset event occurred. As a result, the conversion price of the YA II PN Note was reduced to $0.34 per share of common stock. This was considered a material modification of the note; the remaining balance of the discount to the note in the amount of $699,628 was charged to interest expense, a new discount in the amount of $750,000 was charged to additional paid-in capital, and $620,052 of the new discount was amortized to interest expense. During the year ended May 31, 2019, the Company accrued interest expense in the amount of $36,274 on the YA II PN Note. During the year ended May 31, 2019, the holder of the YA II PN Note converted principal in the amount of $500,000 and accrued interest in the amount of $36,274 into 1,340,684 shares of common stock. Also during the year ended May 31, 2019, the Company made principal and interest payments in the amount of $250,000 and $2,630, respectively, along with a prepayment penalty in the amount of $62,500 on the YA II PN Note. |
- | 750,000 | ||||||
|
Unsecured convertible note issued to Jay Lasky (the “Lasky Note”), dated May 3, 2018 in the original principal amount of $25,000. This note bears interest at the rate of 10% per annum. No interest payments are required until July 1, 2019, at which time all accrued interest becomes due and payable. Commencing on October 1, 2019, the first of eight principal payments in the amount of $3,125 will become due; subsequent payments will become due on the first day of each January, April, July and October until paid in full. The Lasky Note and accrued interest under the note may be converted, in whole or in part, into one share of common stock for each $0.40 converted. The Company recognized a discount of $7,301 on the Lasky Note related to the beneficial conversion feature at the time of issuance. During the year ended May 31, 2018, $149 of this discount was charged to operations. During the year ended May 31, 2018, the Company accrued interest in the amount of $192 on this note.
During the year ended May 31, 2019, $7,152 of this discount was charged to operations. Also during the year ended May 31, 2019, the Company accrued interest in the amount of $993 on this note. During the year ended May 31, 2019, the holder of the Lasky Note converted $26,185, of which $25,000 was principal and $1,185 was accrued interest, into 65,462 shares of common stock. |
- | 25,000 | ||||||
|
May 31, 2019 |
May 31, 2018 |
|||||||
|
Convertible promissory note payable to YA II PN, Ltd. (the “YA II PN Note 2”) dated July 20, 2018 and bearing interest at a rate of 8% per annum. The lender loaned the Company $500,000, and the note is due on November 14, 2019. YA II PN may, at its option, convert all or a portion of the YA II PN Note 2 and accrued but unpaid interest into shares of common stock at a conversion price of $0.40 per share. The YA II PN Note 2 also contains a reset feature, whereby, absent certain exceptions, if the Company issues equity securities at an effective price less than $0.40 per share of common stock, the conversion price of the YA II PN Note 2 will be reset to such lower price. The Company recognized a discount of $362,500 related to the beneficial conversion feature at the time of issuance. Commencing on December 1, 2018, absent certain exceptions, the first of eight payments in the amount of 62,500 will become due; subsequent payments will become due on the first day of each of the following months until paid in full. During the year ended May 31, 2019, $362,500 of this discount was charged to operations. During the year ended May 31, 2019, the Company accrued interest in the amount of $24,438 on this note. Also during the year ended May 31, 2019, the Company made principal and interest payments in the amount of $500,000 and $24,658, respectively, along with a prepayment penalty in the amount of $125,000 on the YA II PN Note 2. |
- | - | ||||||
|
Convertible debenture in the principal amount of $4,000,000 (the “U.S. Convertible Debenture 1”) dated October 31, 2018, which bears interest, payable quarterly, at a rate of 8% per annum, with interest during the first eighteen months following issuance being payable by increasing, on a quarterly basis, the then-outstanding principal amount of the U.S. Convertible Debenture 1. The U.S. Convertible Debenture 1 matures on a date that is three years following issuance. The U.S. Convertible Debenture 1 is convertible into units (the “Convertible Debenture Units”) at a conversion price of $0.80 per Convertible Debenture Unit. Each Convertible Debenture Unit consists of (i) one share of the Company’s common stock, and (ii) one-half of one warrant, with each warrant exercisable for three years to purchase a share of common stock at a price of $1.10. The value of the warrants will be recorded when the issuance becomes probable. The U.S. Convertible Debenture 1 has other features, such as mandatory conversion in the event the common stock trades at a particular price over a specified period of time and required redemption in the event of a “Change in Control” of the Company. The U.S. Convertible Debenture 1 is an unsecured obligation of the Company and ranks pari passu in right of payment of principal and interest with all other unsecured obligations of the Company. The Company recorded a discount in the amount of $3,254,896 on the U.S. Convertible Debenture 1. During the year ended May 31, 2019, $632,896 of this discount was charged to operations. During the year ended May 31, 2019, the Company accrued interest in the amount of $191,363 on the U.S. Convertible Debenture 1. Also during the year ended May 31, 2019, the Company transferred the amount of $135,306 from accrued interest to principal of the U.S. Convertible Debenture 1. |
4,135,306 | - | ||||||
|
Convertible debenture in the principal amount of $1,000,000 (the “U.S. Convertible Debenture 2”) dated October 31, 2018, which bears interest, payable quarterly, at a rate of 8% per annum, with interest during the first eighteen months following issuance being payable by increasing, on a quarterly basis, the then-outstanding principal amount of the U.S. Convertible Debenture 2. The U.S. Convertible Debenture 2 matures on a date that is three years following issuance. The U.S. Convertible Debenture 2 is convertible into Convertible Debenture Units at a conversion price of $0.80 per Convertible Debenture Unit. Each Convertible Debenture Unit consists of (i) one share of the Company’s common stock, and (ii) one-half of one warrant, with each warrant exercisable for three years to purchase a share of common stock at a price of $1.10. The value of the warrants will be recorded when the issuance becomes probable. The U.S. Convertible Debenture 2 has other features, such as mandatory conversion in the event the common stock trades at a particular price over a specified period of time and required redemption in the event of a “Change in Control” of the Company. The U.S. Convertible Debenture 2 is an unsecured obligation of the Company and ranks pari passu in right of payment of principal and interest with all other unsecured obligations of the Company. The Company recorded a discount in the amount of $813,724 on the U.S. Convertible Debenture 2. During the year ended May 31, 2019, $158,224 of this discount was charged to operations. During the year ended May 31, 2019, the Company accrued interest in the amount of $47,841 on the U.S. Convertible Debenture 2. Also during the year ended May 31, 2019, the Company transferred the amount of $33,827 from accrued interest to principal of the U.S. Convertible Debenture 2. |
1,033,827 | - | ||||||
|
May 31, 2019 |
May 31, 2018 |
|||||||
|
Convertible debenture in the principal amount of $100,000 (the “U.S. Convertible Debenture 3”) dated October 24, 2018, which bears interest, payable quarterly, at a rate of 8% per annum, with interest during the first eighteen months following issuance being payable by increasing, on a quarterly basis, the then-outstanding principal amount of the U.S. Convertible Debenture 3. The U.S. Convertible Debenture 3 matures on a date that is three years following issuance. The U.S. Convertible Debenture 3 is convertible into Convertible Debenture Units at a conversion price of $0.80 per Convertible Debenture Unit. Each Convertible Debenture Unit consists of (i) one share of the Company’s common stock, and (ii) one-half of one warrant, with each warrant exercisable for three years to purchase a share of common stock at a price of $1.10. The value of the warrants will be recorded when the issuance becomes probable. The U.S. Convertible Debenture 3 has other features, such as mandatory conversion in the event the common stock trades at a particular price over a specified period of time and required redemption in the event of a “Change in Control” of the Company. The U.S. Convertible Debenture 3 is an unsecured obligation of the Company and ranks pari passu in right of payment of principal and interest with all other unsecured obligations of the Company. The Company recorded a discount in the amount of $75,415 on the U.S. Convertible Debenture 3. During the year ended May 31, 2019, $14,664 of this discount was charged to operations. During the year ended May 31, 2019, the Company accrued interest in the amount of $4,945 on the U.S. Convertible Debenture 3. Also during the year ended May 31, 2019, the Company transferred the amount of $3,541 from accrued interest to principal of the U.S. Convertible Debenture 3. |
103,541 | - | ||||||
|
Convertible debenture in the principal amount of $532,000 (the “U.S. Convertible Debenture 4”) dated October 25, 2018, which bears interest, payable quarterly, at a rate of 8% per annum, with interest during the first eighteen months following issuance being payable by increasing, on a quarterly basis, the then-outstanding principal amount of the U.S. Convertible Debenture 4. The U.S. Convertible Debenture 4 matures on a date that is three years following issuance. The U.S. Convertible Debenture 4 is convertible into Convertible Debenture Units at a conversion price of $0.80 per Convertible Debenture Unit. Each Convertible Debenture Unit consists of (i) one share of the Company’s common stock, and (ii) one-half of one warrant, with each warrant exercisable for three years to purchase a share of common stock at a price of $1.10. The value of the warrants will be recorded when the issuance becomes probable. The U.S. Convertible Debenture 4 has other features, such as mandatory conversion in the event the common stock trades at a particular price over a specified period of time and required redemption in the event of a “Change in Control” of the Company. The U.S. Convertible Debenture 4 is an unsecured obligation of the Company and ranks pari passu in right of payment of principal and interest with all other unsecured obligations of the Company. The Company recorded a discount in the amount of $416,653 on the U.S. Convertible Debenture 4. During the year ended May 31, 2019, $81,016 of this discount was charged to operations. During the year ended May 31, 2019, the Company accrued interest in the amount of $26,185 on the U.S. Convertible Debenture 4. Also during the year ended May 31, 2019, the Company transferred the amount of $18,719 from accrued interest to principal of the U.S. Convertible Debenture 4. |
550,719 | - | ||||||
|
Convertible debenture in the principal amount of $150,000 (the “U.S. Convertible Debenture 5”) dated October 26, 2018, which bears interest, payable quarterly, at a rate of 8% per annum, with interest during the first eighteen months following issuance being payable by increasing, on a quarterly basis, the then-outstanding principal amount of the U.S. Convertible Debenture 5. The U.S. Convertible Debenture 5 matures on a date that is three years following issuance. The U.S. Convertible Debenture 5 is convertible into Convertible Debenture Units at a conversion price of $0.80 per Convertible Debenture Unit. Each Convertible Debenture Unit consists of (i) one share of the Company’s common stock, and (ii) one-half of one warrant, with each warrant exercisable for three years to purchase a share of common stock at a price of $1.10. The value of the warrants will be recorded when the issuance becomes probable. The U.S. Convertible Debenture 5 has other features, such as mandatory conversion in the event the common stock trades at a particular price over a specified period of time and required redemption in the event of a “Change in Control” of the Company. The U.S. Convertible Debenture 5 is an unsecured obligation of the Company and ranks pari passu in right of payment of principal and interest with all other unsecured obligations of the Company. The Company recorded a discount in the amount of $120,100 on the U.S. Convertible Debenture 5. During the year ended May 31, 2019, $23,353 of this discount was charged to operations. During the year ended May 31, 2019, the Company accrued interest in the amount of $7,348 on the U.S. Convertible Debenture 5. Also during the year ended May 31, 2019, the Company transferred the amount of $5,244 from accrued interest to principal of the U.S. Convertible Debenture 5. |
155,244 | - | ||||||
|
May 31, 2019 |
May 31, 2018 |
|||||||
|
Convertible debenture payable in the principal amount of $75,000 (the “U.S. Convertible Debenture 6”) dated October 26, 2018, which bears interest, payable quarterly, at a rate of 8% per annum, with interest during the first eighteen months following issuance being payable by increasing, on a quarterly basis, the then-outstanding principal amount of the U.S. Convertible Debenture 6. The U.S. Convertible Debenture 6 matures on a date that is three years following issuance. The U.S. Convertible Debenture 6 is convertible into Convertible Debenture Units at a conversion price of $0.80 per Convertible Debenture Unit. Each Convertible Debenture Unit consists of (i) one share of the Company’s common stock, and (ii) one-half of one warrant, with each warrant exercisable for three years to purchase a share of common stock at a price of $1.10. The value of the warrants will be recorded when the issuance becomes probable. The U.S. Convertible Debenture 6 has other features, such as mandatory conversion in the event the common stock trades at a particular price over a specified period of time and required redemption in the event of a “Change in Control” of the Company. The U.S. Convertible Debenture 6 is an unsecured obligation of the Company and ranks pari passu in right of payment of principal and interest with all other unsecured obligations of the Company. The Company recorded a discount in the amount of $60,049 on the U.S. Convertible Debenture 6. During the year ended May 31, 2019, $11,676 of this discount was charged to operations. During the year ended May 31, 2019, the Company accrued interest in the amount of $3,674 on the U.S. Convertible Debenture 6. Also during the year ended May 31, 2019, the Company transferred the amount of $2,622 from accrued interest to principal of the U.S. Convertible Debenture 6. |
77,622 | - | ||||||
|
Seventy-two convertible debentures payable in the aggregate principal amount of $12,012,000 (the “Canaccord Debentures”) dated December 12, 2018, which bear interest, payable quarterly, at a rate of 8% per annum, with interest during the first eighteen months following issuance being payable by increasing, on a quarterly basis, the then-outstanding principal amount of the Canaccord Debentures. The Canaccord Debentures mature on a date that is three years following issuance. The Canaccord Debentures are convertible into Convertible Debenture Units at a conversion price of $0.80 per Convertible Debenture Unit. Each Convertible Debenture Unit consists of (i) one share of the Company’s common stock, and (ii) one-half of one warrant, with each warrant exercisable for three years to purchase a share of common stock at a price of $1.10. The value of the warrants will be recorded when the issuance becomes probable. The Canaccord Debentures have other features, such as mandatory conversion in the event the common stock trades at a particular price over a specified period of time and required redemption in the event of a “Change in Control” of the Company. The Canaccord Debentures are unsecured obligations of the Company and rank pari passu in right of payment of principal and interest with all other unsecured obligations of the Company. The Company recorded a discount in the amount of $2,938,690 on the Canaccord Debentures. During the year ended May 31, 2019, $456 of this discount was charged to operations. During the year ended May 31, 2019, the Company accrued interest in the amount of $458,759 on the Canaccord Debentures Also during the year ended May 31, 2019, the Company transferred the amount of $291,791 from accrued interest to principal of the Canaccord Debentures. |
12,303,971 | - | ||||||
|
Total - Convertible Notes Payable |
$ | 18,360,230 | $ | 1,380,000 | ||||
|
Less: Discount |
(3,819,010 |
) |
(1,295,527 |
) |
||||
|
Convertible Notes Payable, Net of Discounts |
$ | 14,541,220 | $ | 84,473 | ||||
|
Total - Convertible Notes Payable, Net of Discounts, Current Portion |
$ | - | $ | 43,401 | ||||
|
Total - Convertible Notes Payable, Net of Discounts, Long-term Portion |
$ | 14,541,220 | $ | 41,072 | ||||
|
Discounts on notes payable amortized to interest expense – years ended May 31, 2019 and 2018, respectively |
$ | 3,576,161 | $ | 2,534,104 |
Aggregate maturities of notes payable and convertible notes payable as of May 31, 2019 are as follows:
For the twelve months ended May 31,
|
2020 |
$ | 4,000,000 | ||
|
2021 |
- | |||
|
2022 |
18,360,230 | |||
|
2023 |
- | |||
|
2024 |
- | |||
|
Thereafter |
- | |||
|
Total |
$ | 22,360,230 |
Beneficial Conversion Features
The Darling Note, Efrat Note, Lamadrid Note and YA II PN Notes contained conversion features that create derivative liabilities. The pricing model the Company uses for determining fair value of its derivatives is the Lattice Model. Valuations derived from this model are subject to ongoing internal and external verification and review. The model uses market-sourced inputs such as interest rates and stock price volatilities. Selection of these inputs involves management’s judgment and may impact net income. The derivative components of the notes were valued at issuance, at conversion, at restructure, and at each period end.
On June 1, 2018, the Company adopted ASU 2017-11 and accordingly reclassified the fair value of the reset provisions embedded in convertible notes payable and certain warrants with embedded anti-dilutive provisions from liability to equity in the aggregate amount of $1,265,751. See note 3.
Certain of the Company’s other convertible notes payable contain beneficial conversion features that are not derivatives, but which require valuation in order to determine the discount to the related convertible note payable. The value of these conversion features is calculated using the intrinsic value method, whereby the amount of the discount is calculated as the difference between the conversion price and the market price of the underlying common stock at the date of issuance multiplied by the number of shares issuable.
NOTE 16 – CONTINGENT LIABILITY
The terms of the Company’s acquisition of Alternative Solutions, include a payment of $1,000,000 contingent upon the Oasis LLCs achieving certain revenue targets. (see note 4). The fair value of this contingent consideration at the time of the Acquisition Agreement was $678,111 as determined by the Company’s outside valuation consultants. Management has reviewed the value of the contingent consideration, and has concluded that, due to the increased revenue of Alternative Solutions, the fair value of this contingent liability was $1,000,000 at May 31, 2019. The Company recorded a charge to operations in the amount of $321,889 during the year ended May 31, 2019.
NOTE 17 – STOCKHOLDERS’ EQUITY
The Company’s authorized capital stock consists of 750,000,000 and 250,000,000 shares of common stock, par value $0.0001, at May 31, 2019 and 2018, respectively, and 20,000,000 shares of preferred stock, par value $0.001 per share. The Company had 125,839,095 and 50,128,972 shares of common stock issued and outstanding as of May 31, 2019 and 2018, respectively.
The Company recorded imputed interest of $807 and $1,076 during the years ended May 31, 2019 and 2018 on related party payables due to a director and officer of the Company. The Company recorded imputed interest of $807 and $804 during the year ended May 31, 2019 and 2017 on related party payables due to a director and officer of the Company, and charged this amount to additional paid-in capital. During the year ended May 31, 2019, the Company repaid the related party payables in the aggregate amount of $17,930.
Year ended May 31, 2019:
Stock Issued upon Conversion of Notes Payable
On June 12, 2018, Darling Capital, holder of a convertible promissory note, converted a total of $565,000, which consisted of $550,000 of principal and $15,000 of accrued interest, into 1,808,000 shares of common stock.
On August 9, 2018, Efrat Investments, holder of a convertible promissory note, converted a total of $57,200, which consisted of $55,000 of principal and $2,200 of accrued interest, into 183,040 shares of common stock.
On August 21, 2018, David Lamadrid, a former executive officer of the Company and holder of a convertible promissory note, converted a total of $32,497, which consisted of $31,250 of principal and $1,247 of accrued interest, into 103,989 shares of common stock.
On August 23, 2018, Jay Lasky, holder of a convertible promissory note, converted a total of $26,185, which consisted of $25,000 of principal and $1,185 of accrued interest, into 65,462 shares of common stock.
On October 23, 2018, Newcan, which is owned by a director of the Company and is the holder of a convertible promissory note, converted a total of $78,534, which consisted of $75,000 of principal and $3,534 of accrued interest, into 196,336 shares of common stock.
On November 14, 2018, YA II PN, holder of a convertible promissory note, converted a total of $280,247, which consisted of $250,000 of principal and $30,247 of accrued interest, into 700,616 shares of common stock.
On January 8, 2019, YA II PN, holder of a convertible promissory note, converted a total of $256,027 which consisted of $250,000 of principal and $6,027 of accrued interest, into 640,068 shares of common stock.
There were no gains or losses on the conversion of notes payable during the year ended May 31, 2019, as all conversions were made pursuant to the terms of the convertible note agreements.
Stock Issued for Services
On June 24, 2018, pursuant to the terms of a severance agreement between the Company and David Lamadrid, the Company issued 600,000 shares of restricted common stock to Mr. Lamadrid. These shares were valued at $264,000 based upon the Company’s stock price of $0.44 on Mr. Lamadrid’s date of employment; $213,320 of this amount had been previously expensed. The remaining $50,680 was charged to operations during the year ended May 31, 2019.
On July 24, 2018, the Company awarded Star Associates, LLC, a limited liability company owned by Andrew Glashow, a director and executive officer of the Company, a cash payment in the amount of $250,000 and 700,000 shares of the Company’s restricted common stock in recognition of Mr. Glashow’s efforts, through Star Associates, in successfully assisting the Company in negotiating and obtaining the financing necessary to acquire Alternative Solutions. The shares were valued at $490,000 based upon the Company’s stock price of $0.70 at the date of the grant, and were charged to operations during the year ended May 31, 2019.
On September 11, 2018, the Company issued 31,250 shares of common stock valued at $25,310 based upon the Company’s stock price of $0.81 at the date of the grant in exchange for legal services previously rendered to the Company. These shares were accrued on February 8, 2018, and were issued from stock payable.
Stock Issued for Acquisition
On June 27, 2018, the Company issued 22,058,823 shares of its common stock pursuant to the terms of the Alternative Solutions Acquisition Agreement. These shares were valued at $15,441,176. (See note 4).
Special Warrants Issued in Offering
On June 20, 2018, the Company executed an Agency Agreement with Canaccord Genuity Corp. and closed on a private offering of its special warrants for aggregate gross proceeds of C$13,037,859 (USD$9,785,978). Pursuant to the offering, the Company issued 28,973,020 special warrants at a price of C$0.45 (USD$0.34) per special warrant. Each special warrant was automatically exercisable, for no additional consideration, into units of the Company on the earlier of: (i) the date that was five business days following the date on which the Company obtained a receipt from the applicable securities regulatory authorities in each of the jurisdictions in Canada in which the special warrants were sold for a final prospectus qualifying the distribution of the units, which was intended to be no later than November 30, 2018, and (ii) the date that was four months and one day after the completion of the Company's acquisition of all of the membership interests in Alternative Solutions, known as Oasis Cannabis. The Company allocated $4,226,394 of the proceeds from the sale of the special warrants to the underlying stock, and $5,559,584 of the value to the underlying warrants. The value of the warrants underlying the special warrants was determined utilizing the Black-Scholes valuation model. The Company recorded a loss on currency conversion in the amount of $403,588 in connection with the special warrants during the year ended May 31, 2019.
In connection with the offering, the Company paid Canaccord Genuity Corp. a cash commission equal to C$1,043,028 (USD$799,053), a corporate finance fee equal to 1,448,651 special warrants, and 2,317,842 compensation broker warrants valued at $1,495,373. Each compensation broker warrant entitles the holder thereof to acquire one unit at a price of C$0.45 per unit for a period of 36 months from the date that the Company's common stock is listed on a recognized Canadian stock exchange, subject to adjustment in certain events. The 1,448,651 special warrants that were issued were valued at $1,413,300 and were charged to operations during the year ended May 31, 2019.
Upon exercise of the special warrants, each unit was to consist of one share of the Company's common stock and one warrant to purchase one share of common stock. Each warrant was to be exercisable at a price of C$0.65 for three years after the Company's common stock was listed on a recognized Canadian stock exchange, subject to adjustment in certain events.
Because the Company did not receive a receipt from the applicable Canadian securities authorities for the qualifying prospectus by August 20, 2018, the unexercised special warrants were adjusted to entitle the holders to receive 1.1 units instead of one unit of the Company. This resulted in the issuance of an additional 3,042,167 units. This penalty was valued at $7,142,550 and was charged to operations during the year ended May 31, 2019.
On February 28, 2019, all of the special warrants were automatically converted into 33,463,838 shares of common stock and warrants to purchase 33,463,838 shares of common stock for CD$0.65 per share.
Stock Issued in Navy Capital Offering
On July 31, 2018, the Company entered into a Subscription Agreement with Navy Capital Green International, Ltd, (the “Navy Capital Offering”) for 7,500,000 units at a price of $0.40 per unit, or an aggregate amount of $3,000,000. The units collectively represent (i) 7,500,000 shares of common stock, and (ii) three-year warrants to purchase an aggregate of 7,500,000 shares of common stock at an exercise price of $0.60 per share of common stock.
In connection with the Navy Capital Offering, between August 8, 2018 and August 10, 2018, the Company entered into five subscription agreements for a total of 6,875,000 units at a price of $0.40 per unit, or an aggregate purchase price of $2,750,000. The units collectively represent (i) 6,875,000 shares of common stock, and (ii) three-year warrants to purchase an aggregate of 6,875,000 shares of common stock at an exercise price of $0.60 per share of common stock.
Stock Issued to Officers
Effective July 1, 2018, the Company granted the Chief Executive Officer of CLS Nevada, Inc. a one-time signing bonus of 500,000 shares of restricted common stock, which shall become fully vested one year from the effective date of his employment agreement. These shares were valued at $350,000 and will be amortized over the vesting period. As of May 31, 2019, $325,417 had been charged to operations, and is carried as Common Stock Subscribed on the Company’s balance sheet at May 31, 2019.
Effective July 1, 2018, the Company granted the Chief Operating Officer of CLS Nevada, Inc. a one-time signing bonus of 50,000 shares of restricted common stock, which shall become fully vested one year from the effective date of his employment agreement. These shares were valued at $35,000 and will be amortized over the vesting period. As of May 31, 2019, $32,542 had been charged to operations, and is carried as Common Stock Subscribed on the Company’s balance sheet at May 31, 2019.
Effective August 1, 2018, the Company granted 25,000 shares of restricted common stock to its then Chief Financial Officer. These shares vested four months after issuance. The shares were valued at $17,500, and were amortized over the vesting period. On April 11, 2019, these shares were issued.
Effective March 1, 2019, the Company granted its President and Chief Operating Officer Chief Operating Officer 500,000 shares of restricted common stock, which shall become fully vested two years from the effective date of his employment agreement. These shares were valued at $215,500 and will be amortized over the vesting period. As of May 31, 2019, $26,938 had been charged to operations, and is carried as Common Stock Subscribed on the Company’s balance sheet at May 31, 2019.
Effective May 2, 2019, the Company granted its Chief Financial Officer 50,000 shares of restricted common stock, which shall become fully vested one year from the effective date of his employment agreement. These shares were valued at $17,995 and will be amortized over the vesting period. As of May 31, 2019, $1,428 had been charged to operations, and is carried as Common Stock Subscribed on the Company’s balance sheet at May 31, 2019.
Stock Issued upon Cashless Exercise of Warrants
On August 14, 2018, the Company issued 129,412 shares of common stock upon the cashless exercise of warrants to purchase 350,000 shares of common stock at an exercise price of $0.75 per share.
On September 6, 2018, the Company issued 13,684 shares of common stock upon the cashless exercise of warrants to purchase 40,000 shares of common stock at an exercise price of $0.75 per share.
On November 14, 2018, the Company issued 5,867 shares of common stock upon the cashless exercise of warrants to purchase 25,000 shares of common stock at an exercise price of $0.75 per share.
Stock Issued for Settlement
On November 1, 2018, the Company issued 50,000 shares of common stock with a fair value of $47,500 pursuant to a legal settlement. There was no gain or loss associated with this transaction.
Stock Issued for Compensation for Debenture Offering
On December 12, 2018, in connection with the issuance of the Canaccord Debentures, the Company issued 559,750 units as compensation for advisory and agent fees. Each unit is comprised of one share of common stock and one-half of one common stock purchase warrant at an exercise price of $1.10 per whole share of common stock. As a result, the Company issued 559,750 shares of common stock as compensation for agent and advisory services. These shares were valued at $557,335, and this amount was charged to operations during the year May 31, 2019.
Additional Paid-in Capital
During the year ended May 31, 2019, the Company recorded discounts on two convertible notes payable related to the beneficial conversion features in the amounts of $362,500 on the YA II PN Note 2, and $58,594 on the Newcan Convertible Note 8. Also, during the year ended May 31, 2019, a reset event occurred with regard to the YA II PN 2 Note.
During the year ended May 31, 2019, the Company recorded an original issue discount on the Oasis Note in the amount of $189,180.
On June 1, 2018, the Company adopted ASU 2017-11 and accordingly reclassified the fair value of the reset provisions embedded in the previously issued convertible notes payable and certain warrants with embedded anti-dilutive provisions from liability to additional paid-in capital in the aggregate amount of $1,265,751. On June 20, 2018, a reset event occurred in connection with the YA II PN 2 Note (see note 15), and the Company charged the change in fair value of the conversion feature in the amount of $35,833 to additional paid-in capital. This was considered a material modification of the note, and the Company created a new discount to this note in the amount of $750,000, which was charged to additional paid-in capital.
During the year ended May 31, 2019, the Company recorded discounts on six convertible debentures related to the beneficial conversion features as follows: a discount of $3,254,896 was recorded on U.S. Convertible Debenture 1; a discount of $813,724 was recorded on U.S. Convertible Debenture 2; a discount of $75,415 was recorded on U.S. Convertible Debenture 3; a discount of $416,653 was recorded on U.S. Convertible Debenture 4; a discount of $120,100 was recorded on U.S. Convertible Debenture 5; and a discount of $60,049 was recorded on U.S. Convertible Debenture 6.
Warrants
On June 27, 2018, the Company incurred a penalty in connection with the WestPark Offering due to the late filing of the registration statement that included the resale of the securities that were sold in such offering. As a result of the penalty, the Company issued three-year common stock warrants to purchase an aggregate of 1,368,250 shares of the Company’s common stock at an exercise price of $0.50 per share. In addition, the Company reduced the exercise price of the common stock purchase warrants previously issued to the investors in the WestPark Offering from $0.75 per share to $0.50 per share. The fair value of the penalty was $941,972; this amount was charged to operations during the three months ended August 31, 2018.
On July 20, 2018, in connection with the Company’s sale of a convertible debenture, the Company issued to YA II PN, Ltd. a five-year common stock purchase warrant to purchase 1,250,000 shares of the Company’s common stock at an initial exercise price of $0.60 per share.
On August 6, 2018, the Company issued three-year common stock purchase warrants to purchase an aggregate of 7,500,000 shares of the Company’s common stock at an exercise price of $0.60 per share, to investors in the Navy Capital Offering.
On August 8, 2018, the Company issued three-year common stock purchase warrants to purchase an aggregate of 6,875,000 shares of the Company’s common stock at an exercise price of $0.60 per share, to investors in the Navy Capital Offering.
Unit Warrants
On June 20, 2018, in connection with the special warrant offering, the Company issued Canaccord Genuity Corp. 2,317,842 three-year broker warrants at an exercise price of C$0.45 per share as compensation. Each warrant entitles the holder to purchase one unit, which consists of one share of common stock and a warrant to purchase one share of common stock, for C$0.65 per share. These warrants were valued at $1,495,373, and this amount was charged to operations during the three months ended August 31, 2018.
On December 12, 2018, in connection with the issuance of the Canaccord Debentures, the Company issued Canaccord Genuity Corp. as compensation 1,074,720 three-year agent and advisory warrants. Each warrant entitles the holder to purchase a unit for $0.80, which unit consists of one share of common stock and a warrant to purchase one-half share of common stock at an exercise price of $1.10 per share. The Company, in connection with the issuance of the Canaccord Debentures, also issued to National Bank Financial Inc., as compensation, 268,680 three-year agent and advisory warrants. Each warrant entitles the holder to purchase a unit for $0.80, which unit consists of one share of common stock and a warrant to purchase one-half share of common stock at an exercise price of $1.10 per share. The aggregate value of these warrants was $874,457, which was charged to operations during the year ended May 31, 2019.
Special Warrants
On June 20, 2018, the Company sold 28,973,019 special warrants for net proceeds of US$9,785,978. Each special warrant was automatically exercisable, for no additional consideration, for units of the Company on the earlier of: (i) the date that was five business days following the date on which the Company obtained a receipt from the applicable securities regulatory authorities in each of the jurisdictions in Canada in which the special warrants were sold for a final prospectus qualifying the distribution of the units, which was intended to be no later than November 30, 2018, and (ii) the date that was four months and one day after the completion of the Company's acquisition of all of the membership interests in Alternative Solutions, known as Oasis Cannabis, which was June 28, 2018. The Company allocated $4,226,394 of the proceeds from the sale of the special warrants to the underlying stock, and $5,559,584 of the value to the underlying warrants. The value of the warrants underlying the special warrants was determined utilizing the Black-Scholes valuation model. The Company recorded a loss on currency conversion in the amount of $403,588 in connection with the special warrants during the year ended May 31, 2019.
Upon exercise of the special warrants, each unit was to consist of one share of the Company's common stock and one warrant to purchase one share of common stock. Each warrant was to be exercisable at a price of C$0.65 for three years after the Company's common stock was listed on a recognized Canadian stock exchange, subject to adjustment in certain events.
Because the Company did not receive a receipt from the applicable Canadian securities authorities for the qualifying prospectus by August 20, 2018, the unexercised special warrants were adjusted to entitle the holders to receive 1.1 units instead of one unit of the Company. This resulted in the issuance of an additional 3,042,167 units. This penalty was valued at $7,142,550 and was charged to operations during the year ended May 31, 2019.
On February 28, 2019, all of the special warrants were automatically converted into 33,463,838 shares of common stock and warrants to purchase 33,463,838 shares of common stock for CD$0.65 per share.
The Company valued warrants using the Black-Scholes valuation model utilizing the following variables:
|
May 31, |
May 31, |
|||||||
|
2019 |
2018 |
|||||||
|
Volatility |
79.02 to 400.3 |
% |
99.36 to 99.53 |
% |
||||
|
Dividends |
$ | 0 | $ | 0 | ||||
|
Risk-free interest rates |
2.68% to 2.77 |
% |
2.52% to 2.65 |
% |
||||
|
Term (years) |
3 | 3 | ||||||
Year ended May 31, 2018:
Stock Issued for Services
On July 13, 2017, the Company issued 24,000 shares of common stock to a consultant in exchange for a $6,000 accrued liability for services previously provided. This resulted in a gain on the settlement of accounts payable in the amount of $3,480.
On March 2, 2018, the Company issued 350,000 shares of common stock to a consultant pursuant to the terms of a consulting agreement. The shares issued for services were valued on the date of grant at $261,800.
On February 8, 2018, the Company agreed to issue 31,250 shares of common stock to a consultant. The shares were valued at $25,313, and are recorded on the balance sheet as stock payable. These shares were issued in August, 2018.
During the year ended May 31, 2018, the Company agreed to issue 600,000 shares of common stock to an officer. These shares were valued at $213,321, and are recorded on the balance sheet as stock payable.
Stock Issued upon Note Conversions
On March 12, 2018, pursuant to the Omnibus Loan Agreement, related party convertible noteholders converted principal and interest in the aggregate amount of $1,421,356 and $197,090, respectively, into a total of 5,179,028 shares of common stock.
Stock Issued for Note Exchange
On September 20, 2017, the Company entered into an Exchange Agreement, whereby it agreed to exchange a note issued in April 2015 for 1,500,000 shares of its common stock valued at $510,000. The holder of the April 2015 Note had previously sold it for $105,219, which represented the balance due by the Company, to StarForce Media, Inc., an entity that is not affiliated with the Company. The Company recognized a loss on this exchange in the amount of $404,082, which was charged to operations during the year ended May 31, 2018.
On September 25, 2017, the Company entered into an Exchange Agreement, whereby it agreed to exchange a note that accrued interest at 8% per annum for 4,500,000 shares of its common stock valued at $1,844,035. The Company recognized a loss on this exchange in the amount of $989,032, which was charged to operations during the year ended May 31, 2018.
Stock Issued with Note
On November 15, 2017, the Company issued 250,000 shares of restricted Common Stock, valued at $95,000, as a commitment fee to a convertible note holder.
Stock Issued in Offering
On December 7, 2017, the Company commenced a private offering of its securities, the terms of which were amended on January 17, 2018 (the “WestPark Offering”). The Company offered for sale a minimum of 800,000 units and a maximum of 4,000,000 units at a price of $1.25 per unit. Each unit consisted of four shares of common stock and one warrant to purchase common stock at $0.75 per share.
On February 7, 2018, the Company received gross proceeds of $1,087,500 from the WestPark Offering, of which $146,975 were expenses, resulting in net proceeds of $940,525, from the sale of 870,000 units.
On February 21, 2018, the Company received additional gross proceeds of $100,000 from the WestPark Offering, of which $28,100 were expenses, resulting in net proceeds of $71,900, from the sale of 80,000 units.
On February 28, 2018, the Company received additional gross proceeds of $81,250 from the WestPark Offering, of which $12,148 were expenses, resulting in net proceeds of $69,102, from the sale of 65,000 units.
On March 29, 2018, the Company received additional gross proceeds of $441,563 from the WestPark Offering, of which $62,172 were expenses, resulting in net proceeds of $379,390, from the sale of 353,250 units.
During the year ended May 31, 2018, the Company incurred offering costs of $249,397. The offering costs were charged to additional paid in capital during the year ended May 31, 2018.
Warrants
On November 15, 2017, in connection with the Company’s sale of a convertible debenture, the Company issued FirstFire Global Opportunities Fund, LLC (“FirstFire”) a three-year common stock purchase warrant to purchase 350,000 shares of the Company’s common stock at an initial exercise price of $0.75 per share. These warrants were valued at $123,950 and were charged to operations during the twelve months ended May 31, 2018.
On February 7, 2018 in connection with the WestPark Offering, the Company issued 870,000 warrants to purchase one share of common stock each at a price of $0.75 per share.
On February 9, 2018, in connection with the Company’s sale of a convertible debenture, the Company issued Darling Capital, LLC (“Darling”) a three-year common stock purchase warrant to purchase 400,000 shares of the Company’s common stock at an initial exercise price of $0.75 per share. These warrants were valued at $313,128 and were charged to operations during the twelve months ended May 31, 2018.
On February 16, 2018, in connection with the Company’s sale of a convertible debenture, the Company issued Efrat Investments, LLC (“Efrat”) a three-year common stock purchase warrant to purchase 40,000 shares of the Company’s common stock at an initial exercise price of $0.75 per share. These warrants were valued at $32,076 and were charged to operations during the twelve months ended May 31, 2018.
On February 21, 2018 in connection with the WestPark Offering, the Company issued 80,000 warrants to purchase one share of common stock each at a price of $0.75 per share.
On February 26, 2018, in connection with the Company’s sale of a convertible debenture, the Company issued David Lamadrid, a former executive officer of the Company, a three-year common stock purchase warrant to purchase 25,000 shares of the Company’s common stock at an initial exercise price of $0.75 per share. These warrants were valued at $18,794 and were charged to operations during the twelve months ended May 31, 2018.
On February 28, 2018 in connection with the WestPark Offering, the Company issued 65,000 warrants to purchase one share of common stock each at a price of $0.75 per share.
On March 2, 2018, the Company issued three-year common stock purchase warrants to purchase an aggregate of 412,500 shares of the Company’s common stock at an exercise price of $0.75 per share to consultants. These warrants were value at $294,173 and were changed to operations during the twelve months ended May 31, 2018.
On March 29, 2018, in connection with the WestPark Offering, the Company issued 65,000 warrants to purchase one share of common stock each at a price of $0.75 per share.
On May 9, 2018, in connection with the Amendment to the FirstFire Note, the Company amended the FirstFire three-year common stock purchase warrant to provide that the holder could purchase an additional 25,000 shares of the Company’s common stock at an initial exercise price of $0.75 per share. These additional warrants were valued to $15,977 and were charged to operations during the twelve months ended May 31, 2018.
On May 14, 2018, in connections with the Company’s sale of a convertible debenture, the Company issued YA II PN, Ltd. a five-year common stock purchase warrant to purchase 1,875,000 shares of the Company’s common stock at an initial exercise price of $0.60 per share. These warrants were valued at $1,300,545 and were charged to operations during the twelve months ended May 31, 2018.
Unit Warrants
As of May 31, 2018, the Company had issued to WestPark Capital, Inc., the placement agent for the WestPark Offering, a five-year warrant to purchase 205,238 of the Company’s units at an exercise price of $1.25 per unit (the “Unit Warrants”). Each unit consists of four shares of common stock and one warrant to purchase a share of common stock for $0.75 per share. The Unit Warrants are part of the placement agent’s compensation pursuant to the placement agent agreement. The Unit Warrants were valued at $503,655, which amount was charged to operations during the year ended May 31, 2018.
During the year ended May 31, 2019, in connection with the Special Warrant Offering, the Company issued 2,317,842 broker warrants. Each broker warrant entitles the holder thereof to acquire one unit at a price of CD$0.45 per unit for a period of 36 months from the date that our common stock is listed on a recognized Canadian stock exchange, subject to adjustment in certain events. Each unit consists of one share of Common Stock, and one warrant to purchase a share of Common Stock.
During the year ended May 31, 2019, in connection with the issuance of the Canaccord Debentures, the Company issued Canaccord Genuity Corp., as compensation, 1,074,720 three-year agent and advisory warrants. Each warrant entitles the holder to purchase a unit for $0.80, which unit consists of one share of Common Stock and a warrant to purchase one-half share of Common Stock at an exercise price of $1.10 per share. The Company, in connection with the issuance of the Canaccord Debentures, also issued to National Bank Financial Inc., as compensation, 268,680 three-year agent and advisory warrants. Each warrant entitles the holder to purchase a unit for $0.80, which unit consists of one share of Common Stock and a warrant to purchase one-half share of Common Stock at an exercise price of $1.10 per share. The aggregate value of these warrants was $874,457, which was charged to operations during the year ended May 31, 2019.
Additional Paid-in-Capital
During the year ended May 31, 2018, the Company recorded discounts on convertible notes payable related to the beneficial conversion feature in the amount of $1,758,741.
During the year ended May 31, 2018, the Company recorded a settlement of derivative liabilities in the amount of $442,775.
The following table summarizes the significant terms of warrants outstanding at May 31, 2019. This table does not include the unit warrants. See Unit Warrants section below.
|
Range of exercise Prices |
|
|
Number of warrants Outstanding |
|
|
Weighted average remaining contractual life (years) |
|
|
Weighted average exercise price of outstanding Warrants |
|
|
Number of warrants Exercisable |
|
|
Weighted average exercise price of exercisable Warrants |
|
||||||
|
$ |
0.49 |
|
|
|
33,465,110 |
|
|
|
2.50 |
|
|
$ |
0.49 |
|
|
|
33,465,110 |
|
|
$ |
0.49 |
|
|
|
0.50 |
|
|
|
2,736,500 |
|
|
|
2.73 |
|
|
|
0.50 |
|
|
|
2,736,500 |
|
|
|
0.50 |
|
|
|
0.60 |
|
|
|
17,500,000 |
|
|
|
2.50 |
|
|
|
0.60 |
|
|
|
17,500,000 |
|
|
|
0.60 |
|
|
|
0.75 |
|
|
|
837,500 |
|
|
|
1.73 |
|
|
|
0.75 |
|
|
|
837,500 |
|
|
|
0.75 |
|
|
|
1.10 |
|
|
|
279,875 |
|
|
|
2.54 |
|
|
|
1.10 |
|
|
|
279,875 |
|
|
|
1.10 |
|
|
|
|
|
|
|
54,818,985 |
|
|
|
2.50 |
|
|
$ |
0.53 |
|
|
|
54,818,985 |
|
|
$ |
0.53 |
|
Transactions involving warrants are summarized as follows. This table does not include the special warrants or unit warrants. See Special Warrants and Unit Warrants sections below.
|
Number of Shares |
Weighted Average Exercise Price |
|||||||
|
Warrants outstanding at May 31, 2017 |
- | $ | - | |||||
|
Granted |
4,495,750 | $ | 0.61 | |||||
|
Exercised |
- | $ | - | |||||
|
Cancelled / Expired |
- | $ | - | |||||
|
Warrants outstanding at May 31, 2018 |
4,495,750 | $ | 0.61 | |||||
|
Granted |
50,738,235 | $ | 0.53 | |||||
|
Exercised |
(415,000 |
) |
$ | 0.75 | ||||
|
Cancelled / Expired |
- | $ | - | |||||
|
Warrants outstanding at May 31, 2019 |
54,818,985 | $ | 0.53 | |||||
Special Warrants
On June 20, 2018, the Company sold 28,973,019 special warrants (see Special Warrants section above). Each special warrant was exercisable at no additional charge to acquire one share of the Company’s Common Stock and one three-year warrant to purchase one share of Common Stock at a price of C$0.65. All of the special warrants were exercised during the year ended May 31, 2019. Because the special warrants were exercisable for Common Stock and warrants, they were not included in the warrant tables above. There were no special warrants outstanding at May 31, 2018 or 2019.
Unit Warrants
During the year ended May 31, 2018, the Company issued five-year warrants to purchase 205,238 of the Company’s units at an exercise price of $1.25 per unit. Each unit consists of four shares of common stock and one warrant to purchase a share of common stock for $0.75 per share.
During the year ended May 31, 2019, the Company issued 2,317,842 broker warrants. Each broker warrant entitles the holder thereof to acquire one unit at a price of CD$0.45 per unit for a period of 36 months from the date that our Common Stock is listed on a recognized Canadian stock exchange, subject to adjustment in certain events. Each unit consists of one share of Common Stock, and one warrant to purchase a share of Common Stock.
During the year ended May 31, 2019, in connection with the issuance of the Canaccord Debentures, the Company issued an aggregate of 1,343,400 three-year compensation warrants. Each compensation warrants warrant entitles the holder to purchase a unit for $0.80, which unit consists of one share of Common Stock and a warrant to purchase one-half share of Common Stock at an exercise price of $1.10 per share.
Because the unit warrants are exercisable for Common Stock and warrants, they are not included in the warrant tables above.
NOTE 18 – RELATED PARTY TRANSACTIONS
As of May 31, 2019 and 2018, the Company owed the amount of $0 and $37,500, respectively, to Jeffrey Binder, its Chief Executive Officer, for accrued salary. For the twelve months ended May 31, 2018, unpaid accrued salary in the amount of $150,000 was transferred to a convertible promissory note due to Mr. Binder.
As of May 31 2019 and 2018, the Company had accrued salary due to Michael Abrams, a former officer of the Company prior to his September 1, 2015 termination, in the amount of $16,250.
As of May 31, 2019 and 2018, the Company had related party payables in the amount of $0 and $17,930, respectively, due to officers and directors related to expenses paid on behalf of the Company. The Company imputed interest at the rate of 6% per annum on these liabilities, and recorded imputed interest expense on these liabilities in the amounts of $807 and $1,076 during the years ended May 31, 2019 and 2018, respectively. These interest accruals were charged to additional paid-in capital. The Company repaid the amount of $17,930 to the officers and directors effective March 1, 2019.
On July 27, 2018, the Company granted 25,000 shares of restricted common stock to Frank Tarantino, its former Chief Financial Officer. These shares vested four months after issuance. The shares were valued at $17,500, and were amortized over the vesting period. These shares were issued on April 11, 2019.
On July 31, 2018, the Company granted the Chief Executive Officer of CLS Nevada, Inc. a one-time signing bonus of 500,000 shares of restricted common stock, which shall become fully vested one year from the effective date of his employment agreement. These shares were valued at $355,000 and will be amortized over the vesting period. As of May 31, 2019, $325,417 had been charged to operations.
On July 31, 2018, the Company granted the Chief Operating Officer of CLS Nevada, Inc. a one-time signing bonus of 50,000 shares of restricted common stock, which shall become fully vested one year from the effective date of his employment agreement. These shares were valued at $35,000 and will be amortized over the vesting period. As of May 31, 2019, $32,542 had been charged to operations.
On July 24, 2018, the Company awarded Star Associates, LLC, a limited liability company owned by Andrew Glashow, a director of the Company, a cash payment in the amount of $250,000 and 700,000 shares of restricted common stock in recognition of Mr. Glashow’s efforts, through Star Associates, in successfully assisting the Company in negotiating and obtaining the financing necessary to acquire Alternative Solutions. The shares were valued at $490,000 and were charged to operations during the year ended May 31, 2019.
Related Party Notes Payable
On March 12, 2018, the Company received conversion notices from Jeffrey I. Binder, Frank Koretsky, Newcan Investment Partners LLC and CLS CO 2016, LLC (collectively, the “Insiders”). Pursuant to the terms of the conversion notices, the following amounts of principal and accrued interest were converted to common stock of the Company:
|
Accrued |
||||||||||||
|
Principal |
Interest |
# Shares |
||||||||||
|
Jeffrey Binder |
$ | 464,698 | $ | 43,058 | (1,624,819 |
) |
||||||
|
Frank Koretsky |
- | 46,626 | (149,203 |
) |
||||||||
|
Newcan Investment Partners LLC |
956,658 | 98,098 | (3,375,220 |
) |
||||||||
|
CLS CO 2016 LLC |
- | 9,308 | (29,786 |
) |
||||||||
|
Total |
$ | 1,421,356 | $ | 197,090 | (5,179,028 |
) |
||||||
At May 31, 2018, the Company had $143,887 in principal and $5,142 in accrued interest of convertible notes payable outstanding to Jeffrey Binder, an officer and director, David Lamadrid, an officer, and to Newcan Investment Partners, LLC, an entity wholly owned by Frank Koretsky, a director.
During the year ended May 31, 2019, the Company made principal and interest payments to Mr. Binder in the amount of $37,500 and $3,903, respectively. At May 31, 2019, the Company had no principal or accrued interest payable to Mr. Binder.
During the year ended May 31, 2019, David Lamadrid converted principal in the amount of $31,250 and accrued interest in the amount of $1,247 into a total of 103,989 shares of common stock. At May 31, 2019, the Company had no principal or accrued interest payable to Mr. Lamadrid.
During the year ended May 31, 2019, Newcan Investment Partners, LLC, converted principal in the amount of $75,000 and accrued interest in the amount of $3,534 into a total of 196,336 shares of common stock. At May 31, 2019, the Company had no principal or accrued interest payable to Newcan Investment Partners, LLC.
NOTE 19 – INCOME TAXES
The Company accounts for income taxes under FASB ASC 740-10, which provides for an asset and liability approach of accounting for income taxes. Under this approach, deferred tax assets and liabilities are recognized based on anticipated future tax consequences, using currently enacted tax laws, attributed to temporary differences between the carrying amounts of assets and liabilities for financial reporting purposes and the amounts calculated for income tax purposes.
As of May 31, 2019 and 2018, the Company had incurred a net operating loss and, accordingly, no provision for income taxes has been recorded. In addition, no benefit for income taxes has been recorded due to the uncertainty of the realization of any tax assets.
The Company’s tax rate was reduced from 34% for the year ended May 31, 2018 to 21% for the year ended May 31, 2019 due to changes in the federal income tax rate enacted by the 2018 Tax Cuts and Jobs Act. The tax effects of the temporary differences that give rise to the Company’s estimated deferred tax assets and liabilities are as follows:
|
May 31, |
May 31, |
|||||||
|
2019 |
2018 |
|||||||
|
Federal and state statutory rate |
21 |
% |
34 |
% |
||||
|
Net operating loss carry forwards |
3,802,491 | 2,790,481 | ||||||
|
Valuation allowance for deferred tax assets |
(3,802,491 |
) |
(2,790,481 |
) |
||||
|
Net deferred tax assets |
- | - | ||||||
As of May 31, 2019 and 2018, the Company had net operating loss carry forwards of approximately $3,802,491 and $2,790,481 available to offset future taxable income. The net operating loss carry forwards, if not utilized, will begin to expire in 2037.
Based on the available objective evidence, including the Company’s history of losses, management believes it is more likely than not that the net deferred tax assets will not be fully realized. Accordingly, the Company has provided for a full valuation allowance against its net deferred tax assets at May 31, 2019 and 2018. The Company had no uncertain tax positions as of May 31, 2019.
NOTE 20 – COMMITMENTS AND CONTINGENCIES
Lease Arrangements
The Company leases several facilities for office, warehouse, and retail space. Currently lease commitments are as follows:
An eighteen month lease, which commenced February, 2019, for 1,400 square feet of office space located at 1718 Industrial Road, Las Vegas, NV 89102 for the initial amount of $1,785 per month, increasing to $1,887 per month;
A five-year lease, which commenced July, 2014 and was extended to June 30, 2023, for 1,000 square feet of storefront plus 5,900 square feet of warehouse space located at 1800 Industrial Road, Suites 102, 160, and 180, Las Vegas, NV 89102 for the initial amount of $7,500 per month, increasing to $9,501 per month;
An eighteen month lease, which commenced February, 2019, for 2,504 square feet of office space located at 1800 Industrial Road, Suite 100, Las Vegas, NV 89102 for the initial amount of $3,210 per month, increasing to $3,339 per month;
A five-year lease, which commenced January, 2016, for 22,000 square feet of warehouse space located at 203 E. Mayflower Avenue, North Las Vegas, NV 89030 for the initial amount of $11,000 per month, increasing to $29,000 per month.
Five-year minimum lease payments under current lease agreements as of May 31, 2019 are as follows:
For the twelve months ended May 31,
|
2020 |
$ |
485,030 |
||
|
2021 |
379,092 |
|||
|
2022 |
110,459 |
|||
|
2023 |
113,735 |
|||
|
2024 |
9,501 |
|||
|
Thereafter |
- |
|||
|
Total |
$ |
1,097,817 |
In connection with the Company’s planned Colorado operations, on April 17, 2015, pursuant to an Industrial Lease Agreement (the “Lease”), CLS Labs Colorado leased 14,392 square feet of warehouse and office space (the “Leased Real Property”) in a building in Denver, Colorado where certain intended activities, including growing, extraction, conversion, assembly and packaging of cannabis and other plant materials, are permitted by and in compliance with state, city and local laws, rules, ordinances and regulations. The Lease had an initial term of seventy-two (72) months and provided CLS Labs Colorado with two options to extend the term of the lease by up to an aggregate of ten (10) additional years. In August 2017, as a result of the Company’s decision to suspend its proposed operations in Colorado, CLS Labs Colorado asked its landlord to be relieved from its obligations under the Lease, but the parties have not yet reached an agreement on how to proceed.
In August 2017, the Company’s Colorado subsidiary received a demand letter from its Colorado landlord requesting the forfeiture of the $50,000 security deposit, $10,000 in expenses, $15,699 in remaining rent due under the lease agreement and $30,000 to buy out the remaining amounts due under the lease. These expenses, which are a liability of the Company’s Colorado subsidiary, have been accrued on the balance sheet as of May 31, 2019.
Contingent Liability
At the time of closing of the Acquisition Agreement, Alternative Solutions owed certain amounts to a consultant known as 4Front Advisors, which amount was in dispute. In August 2019, we made a payment to this company to settle this dispute and the Oasis Note was reduced accordingly.
Employment Agreements
CLS Labs and Jeffrey Binder entered into a five-year employment agreement effective October 1, 2014. Under the agreement, Mr. Binder serves as CLS Labs’ Chairman and Chief Executive Officer and is entitled to receive an annual salary of $150,000. Under the agreement, Mr. Binder is also entitled to receive a performance bonus equal to 2% of CLS Labs’ annual EBITDA, up to a maximum annual cash compensation of $1 million (including his base salary), and annual stock options, exercisable at the fair market value of CLS Labs’ common stock on the date of grant, in an amount equal to 2% of its annual EBITDA up to $42.5 million and 4% of its annual EBITDA in excess of $42.5 million. On April 28, 2015, CLS Labs and the Company entered into an addendum to Mr. Binder’s employment agreement whereby Mr. Binder agreed that following the merger of CLS Labs and a subsidiary of the Company, in addition to his obligations to CLS Labs, he would serve the Company and its subsidiaries in such roles as the Company may request. In exchange, the Company agreed to assume the obligations of CLS Labs to grant Mr. Binder annual stock options, as referenced above. Mr. Binder continues to receive an annual salary of $150,000 from CLS Labs for serving as its Chairman, President and Chief Executive Officer. On July 20, 2016, March 31, 2017, August 23, 2017, October 9, 2017, January 5, 2018 and April 6, 2018, the Company issued Mr. Binder convertible notes in exchange for $250,000, $112,500, $62,500, $39,521, $37,500 and $37,500 respectively, in deferred salary, among other amounts owed to Mr. Binder by the Company. As of May 31, 2019 and 2018, the Company had accrued compensation due to Mr. Binder in the amounts of $0 and $37,500, respectively.
Effective August 1, 2015, the Company and Alan Bonsett entered into a five-year employment agreement. Pursuant to the agreement, Mr. Bonsett commenced serving as the Company’s Chief Operating Officer on August 15, 2015. Under the agreement, Mr. Bonsett was entitled to receive an annual salary of $150,000. Further, he was entitled to receive a performance bonus equal to 2% of the Company’s annual EBITDA, up to a maximum annual cash compensation of $1 million (including his base salary), and annual stock options, exercisable at the fair market value of the Company’s common stock on the date of grant, in an amount equal to 2% of its annual EBITDA up to $42.5 million and 4% of its annual EBITDA in excess of $42.5 million. Additionally, Mr. Bonsett received a one-time signing bonus of 250,000 (post Reverse-Split) shares of restricted common stock of the Company, valued at $327,500, which became fully vested one year from the effective date of the agreement. Mr. Bonsett, as an owner of Picture Rock Holdings, LLC (“PRH”), was expected to indirectly receive the benefits of the Colorado operations discussed above. Mr. Bonsett agreed to defer his salary effective July 1, 2017; at May 31, 2019, the Company had accrued compensation due to Mr. Bonsett in the amount of $37,500. On October 1, 2017, the Company and Mr. Bonsett, the Company’s Chief Operating Officer, mutually agreed to end his employment with the Company. Mr. Bonsett may provide consulting services to the Company in the future on an as needed basis.
Effective November 30, 2017, the Company and Mr. Lamadrid entered into a one-year employment agreement. Pursuant to the agreement, Mr. Lamadrid commenced serving as the Company’s President and Chief Financial Officer on December 1, 2017. Under the agreement, Mr. Lamadrid was entitled to receive an annual salary of $175,000. Further, he was entitled to receive a performance bonus equal to 2% of the Company’s annual EBITDA, and annual restricted stock awards of the Company’s common stock in an amount equal to 3% of its annual EBITDA. Additionally, Mr. Lamadrid was entitled to a one-time signing bonus of 500,000 shares of restricted common stock of the Company, which were to become fully vested one year from the effective date of the agreement. On July 24, 2018, the Company and Mr. Lamadrid mutually agreed to terminate the employment agreement. Mr. Lamadrid resigned as President and Chief Financial Officer effective as of July 13, 2018. In connection with a severance agreement between the Company and Mr. Lamadrid, the Company paid certain amounts and issued 600,000 shares of common stock to Mr. Lamadrid, and the parties further agreed that neither party would have any further obligations under the Employment Agreement or otherwise after such date.
On July 31, 2018, the Company and Mr. Sillitoe entered into a one-year employment agreement. Pursuant to the agreement, Mr. Sillitoe commenced serving as the Chief Executive Officer of CLS Nevada, Inc. effective July 1, 2018. Under the agreement, Mr. Sillitoe is entitled to receive an annual salary of $150,000. Further, he is entitled to receive a performance bonus equal to 2% of the annual EBITDA of CLS Nevada, Inc., and annual restricted stock awards of the Company’s common stock in an amount equal to 3% of the annual EBITDA of CLS Nevada, Inc. Additionally, Mr. Sillitoe is entitled to a one-time signing bonus of 500,000 shares of restricted common stock, which shall become fully vested one year from the effective date of his employment agreement assuming Mr. Sillitoe remains employed by the Company on such date. On July 31, 2019, CLS Nevada, Inc. and Mr. Sillitoe amended Mr. Sillitoe’s employment agreement to effect the original intention of the parties that the performance bonus would be based on the results of Alternative Solutions and not CLS Nevada, Inc.
The Company and Mr. Decatur entered into a one-year employment agreement effective July 31, 2018. Pursuant to the agreement, Mr. Decatur commenced serving as the Chief Operating Officer of CLS Nevada, Inc. on July 1, 2018. Under the agreement, Mr. Decatur is entitled to receive an annual salary of $150,000. Further, he is entitled to receive a performance bonus equal to 2% of the annual EBITDA of CLS Nevada, Inc., and annual restricted stock awards of the Company’ common stock in an amount equal to 3% of the annual EBITDA of CLS Nevada, Inc. Additionally, Mr. Decatur is entitled to a one-time signing bonus of 50,000 shares of restricted common stock, which shall become fully vested one year from the effective date of his employment agreement assuming Mr. Decatur remains employed by the Company on such date. On May 14, 2019, CLS Nevada and Mr. Decatur entered into an amendment to his employment agreement to extend the term of Mr. Decatur's employment agreement by two years instead of relying on the automatic one-year renewal provision in the employment agreement. On July 31, 2019, CLS Nevada, Inc. and Mr. Decatur amended Mr. Decatur’s employment agreement to effect the original intention of the parties that the performance bonus would be based on the results of Alternative Solutions and not CLS Nevada, Inc.
On May 2, 2019, the Company and Gregg Carlson entered into a one-year employment agreement. Pursuant to the employment agreement, Mr. Carlson commenced serving as the Company’s Chief financial Officer on May 1, 2019 and will continue his employment with us pursuant to the terms of his one-year employment agreement with Alternative Solutions effective April 8, 2019. Mr. Carlson receives an annual salary of $110,000, and received a one-time signing bonus of 50,000 shares of restricted common stock of the Company, which shall become fully vested one year from the effective date of his employment agreement assuming Mr. Carlson remains employed by the Company on such date.
At May 31, 2019 and 2018, the Company had accrued salary due to Michael Abrams, a former officer of the Company, prior to his September 1, 2015 termination, in the amount of $16,250.
NOTE 21 – FAIR VALUE OF FINANCIAL INSTRUMENTS
The following summarizes the Company’s derivative financial liabilities that are recorded at fair value on a recurring basis at May 31, 2019 and 2018.
|
|
|
May 31, 2019 |
|
|||||||||||||
|
|
|
Level 1 |
|
|
Level 2 |
|
|
Level 3 |
|
|
Total |
|
||||
|
Liabilities |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Derivative liabilities |
|
$ |
- |
|
|
$ |
- |
|
|
$ |
- |
|
|
$ |
- |
|
|
|
|
May 31, 2018 |
|
|||||||||||||
|
|
|
Level 1 |
|
|
Level 2 |
|
|
Level 3 |
|
|
Total |
|
||||
|
Liabilities |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Derivative liabilities |
|
$ |
- |
|
|
$ |
- |
|
|
$ |
1,265,751 |
|
|
$ |
1,265,751 |
|
The estimated fair values of the Company’s derivative liabilities are as follows:
|
|
|
Derivative |
|
|
|
|
|
Liability |
|
|
|
Liabilities Measured at Fair Value |
|
|
|
|
|
|
|
|
|
|
|
Balance as of May 31, 2018 |
|
$ |
95,276 |
|
|
|
|
|
|
|
|
Issuances |
|
|
3,671,505 |
|
|
|
|
|
|
|
|
Convert or Redeem |
|
|
(2,696,755 |
) |
|
|
|
|
|
|
|
Revaluation loss |
|
|
195,725 |
|
|
|
|
|
|
|
|
Balance as of May 31, 2018 |
|
$ |
1,265,751 |
|
|
|
|
|
|
|
|
Reclassify to equity pursuant to ASU 2017-11 |
|
|
(1,265,751 |
) |
|
|
|
|
|
|
|
Balance as of May 31, 2019 |
|
$ |
- |
|
NOTE 22 – SUBSEQUENT EVENTS
U.S. Convertible Debentures
On July 26, 2019, the Company, Navy Capital, and two other purchasers of the U.S. Convertible Debentures entered into First Amendments to Convertible Debenture, pursuant to which the parties agreed to adjust the conversion price of the U.S. Convertible Debentures if, in general, the Company issues or sells common stock, or warrants or options exercisable for common stock, or any other securities convertible into common stock, in a capital raising transaction, at a consideration per share, or exercise or conversion price per share, as applicable, less than the conversion price of the U.S. Convertible Debentures in effect immediately prior to such issuance (a “Dilutive Issuance”). In such case, the conversion price of the U.S. Convertible Debentures will be reduced to such issuance price (the “Adjusted Conversion Price”). The amendment also provides that, if a Dilutive Issuance occurs, the warrant to be received upon conversion will be exercisable at a price equal to 137.5% of the Adjusted Conversion Price at the time of conversion of the debenture (the “Revised Warrant Exercise Price”). If a Dilutive Issuance occurs, the form of warrant attached to the subscription agreement shall be amended to change the Initial Exercise Price, as defined therein, to be the Revised Warrant Exercise Price. The remaining terms of the U.S. Convertible Debentures and warrant shall remain in full force and effect.
Common Stock Issued
On July 8, 2019, the Company issued 16,250 shares of common stock and three-year warrants to acquire 8,125 shares of common stock at a price of $1.10 per share to Canaccord Genuity Corp. in connection with the conversion of a portion of the Canaccord Debentures in the principal amount of $13,000.
On July 19, 2019, the Company issued 15,000 shares of common stock and three-year warrants to acquire 7,500 shares of common stock at a price of $1.10 per share to Canaccord Genuity Corp. in connection with the conversion of a portion of the Canaccord Debentures in the principal in the amount of $12,000.
On July 22, 2019, the Company issued 500,000 shares of common stock to Ben Sillitoe, Chief Executive Officer of CLS Nevada, in connection with his employment agreement.
On July 22, 2019, the Company issued 50,000 shares of common stock to Don Decatur, Chief Operating Officer of CLS Nevada, in connection with his employment agreement.
Oasis Note
On August 14, 2019, the Company made a prepayment in the amount of $2,500,000, which was applied to the amount due under the Oasis Note.
Settlement of Liability
On August 14, 2019, the Company made a payment to 4Front Advisors to settle its dispute with Alternative Solutions and its former owners and the Oasis Note was reduced in accordance with its terms. In addition, the amount of $275,000, which the Company had accrued with respect to this dispute, was extinguished.
IGH Option
On August 26, 2019, the Company and In Good Health, Inc., entered into an agreement to extend the Option Period as follows: The Option Period shall mean the period beginning on January 1, 2020 and ending on January 31, 2020, subject to extension.
CannAssist Agreements
On June 24, 2019, the Company advanced the sum of $175,000 to CannAssist, increasing the balance due to the Company under the CannAssist Note to $325,000.
On August 26, 2019, and Company and CannAssist entered into an agreement to amend the CannAssist Note as follows: There will be no additional advances under the note beyond the $150,000 advanced on February 4, 2019, and the $175,000 advanced on June 24, 2019. In addition, the CannAssist note shall become due and payable in full on or before February 28, 2020. In addition, the Company and CannAssist terminated the CannAssist Purchase Agreement.
Item 9. Changes in and Disagreements with Accountants on Accounting and Financial Disclosure.
There have been no disagreements regarding accounting and financial disclosure matters with our independent certified public accountants.
Item 9A. Controls and Procedures.
Evaluation of Disclosure Controls and Procedures
Jeffrey Binder, our Chief Executive Officer, and Gregg Carlson, our Chief Financial Officer, have evaluated the effectiveness of our disclosure controls and procedures (as defined in Rule 13a-15(e) under the Securities Exchange Act of 1934, as amended) as of the end of the period covered by this report. Based on the evaluation, Mr. Binder and Mr. Carlson concluded that our disclosure controls and procedures are not effective in timely alerting them to material information relating to us that is required to be included in our periodic SEC filings and ensuring that information required to be disclosed by us in the reports we file or submit under the Act is accumulated and communicated to our management, including our Chief Financial Officer, or person performing similar functions, as appropriate to allow timely decisions regarding required disclosure, for the following reasons:
● We do not have an independent board of directors or audit committee or adequate segregation of duties;
● We have not established a formal written policy for the approval, identification and authorization of related party transactions
● We do not have an independent body to oversee our internal controls over financial reporting and lack segregation of duties due to our limited resources.
We plan to rectify these weaknesses by implementing an independent board of directors and hiring additional accounting personnel once we have additional resources to do so.
Changes in Internal Control over Financial Reporting
There have been no changes in our internal controls over financial reporting that occurred during our last fiscal quarter that have materially affected, or are reasonably likely to materially affect, our internal control over financial reporting.
None.
PART III
Item 10. Directors, Executive Officers and Corporate Governance.
Our Board of Directors has appointed Jeffrey Binder, Frank Koretsky and Andrew Glashow to serve on the Company’s audit committee. Mr. Glashow serves as chairman of the audit committee. We currently do not have nominating or compensation committees, or committees performing similar functions, nor do we have a written nominating or compensation committee charter. Our Board of Directors does not believe that it is necessary to have such committees because it believes the functions of such committees can be adequately performed by the entire Board of Directors.
Our amended and restated articles of incorporation provide that the Board of Directors be divided into three classes with each class serving a staggered three-year term. The term of Class I directors expired at our 2018 annual meeting, the term of Class II directors expired at our 2016 annual meeting, and the term of Class III directors expired at our 2017 annual meeting. Andrew Glashow serves as the sole member of Class I, Frank Koretsky serves as the sole member of Class II and Jeffrey Binder serves as the sole member of Class III. We did not hold a 2015, 2016, 2017 or 2018 annual meeting due to our desire to conserve cash and focus on financing the Company. As a result, all of our directors are continuing to serve as directors because their successors have not been elected. We anticipate that we will hold an annual meeting during 2019 to elect directors. Executive officers are appointed by the Board of Directors and serve at its pleasure. None of our directors is independent, as that term is defined by Nasdaq rules. None of our directors is a financial expert, as that term is defined by the SEC. To date, the directors and executive officers of the Company, their ages, positions held, and duration as such, are as follows:
|
Name |
|
Age |
|
Title |
|
Term Expires |
|
|
Jeffrey Binder |
|
72 |
|
Chairman, Chief Executive Officer and Director |
|
2017* |
|
|
Frank Koretsky |
|
57 |
|
Director |
|
2016* |
|
|
Andrew Glashow |
|
56 |
|
President, Chief Operating Officer and Director |
|
2018* |
|
|
Gregg Carlson |
|
62 |
|
Chief Financial Officer and Controller of Alternative Solutions |
|
-- |
|
|
Benjamin Sillitoe |
|
42 |
|
Chief Executive Officer of CLS Nevada, Inc. |
|
-- |
|
|
Don Decatur |
|
36 |
|
Chief Operating Officer of CLS Nevada, Inc. |
|
-- |
|
* Messrs. Binder, Koretsky, and Glashow continue to serve as directors until such time as we hold an annual meeting to either re-elect them or elect another person in their stead.
Jeffrey Binder, Chairman, President, Chief Executive Officer and Director
Mr. Binder was one of the individuals who founded CLS Labs in 2014 and he has served as its Chairman, President, Chief Executive Officer and a director since its inception. Upon CLS Labs’ acquiring control of the Company on November 12, 2014, Mr. Binder was appointed Chairman, President, Chief Executive Officer and a director of the Company. He continues to serve in these roles. Since 2008, Mr. Binder has served as founder, Chairman and President of Power 3 Network, Inc., a company that develops websites and back offices for home-based businesses. In 2003, Mr. Binder founded Infinity 8, Inc., a software development company, where he served as its Chairman, Treasurer and a director until 2011. In addition to his employment history, Mr. Binder has invested in and mentored several start-up and mid-stage companies through his private holding company, JeMJ Financial Services, Inc., which he formed in 1988 and for which he serves as Chairman, President and a director. Through JeMJ, Mr. Binder invested in GGL Industries, Inc., a private holding company that owned Sterling Yacht and Classic Motor Carriages, as well as various other companies, and had extensive real estate holdings. Mr. Binder received his Juris Doctorate from the National Law Center, George Washington University, in 1971, where he received the honor of membership in the Order of the Coif. He also served as a legislative assistant to Adlai Stevenson II, a United States Senator for Illinois, and practiced Law at Sonnenschein Nath & Rosenthal, LLP, Chicago, Illinois for five years.
Frank Koretsky, Director
Mr. Koretsky is a founder and has served as a director of CLS Labs since its formation in 2014. Upon consummation of the Merger, Mr. Koretsky was also appointed a director of the Company. It is expected that Mr. Koretsky will serve as a consultant to the Company in the future. Since 1995, Mr. Koretsky has served as the President of East Coast News Corp., a leading company in the adult product distribution industry. As a result of Mr. Koretsky’s business experience, he brings a strong background in management, marketing and branding to the Company.
Andrew Glashow, President, Chief Operating Officer and Director
Mr. Glashow was appointed to serve as our President and Chief Operating Officer commencing on March 1, 2019. Mr. Glashow has served as a partner in Star Associates, LLC, a corporate finance firm specializing in the placement of capital for small and emerging growth companies, since March 2018. Prior to forming Star Associates, Mr. Glashow was a founding partner of New World Merchant Partners LLC, a capital markets and business advisory firm, and served as a Managing Director since its inception in September 2009. Mr. Glashow is an investment banker specializing in microcap transactions in the $5 million to $50 million range. He has in excess of twenty-five years of experience in the capital markets and in all phases of business start-up and growth, including feasibility studies, business plans, equity and debt funding, private placements, reverse mergers and IPOs. Mr. Glashow has worked with many investment banking firms and maintains close relationships with decision makers at several of them. Mr. Glashow has served as CEO and President of multiple companies that he helped capitalize. Mr. Glashow is a graduate of the University of New Hampshire’s Whitemore School of Business and Economics.
Gregg Carlson, Chief Financial Officer and Controller of Alternative Solutions, LLC
Mr. Carlson was appointed to serve as our Chief Financial Officer commencing on May 1, 2019. Between April 8, 2019 and this appointment, Mr. Carlson served as the Controller of Alternative Solutions. Before joining Alternative Solutions, Mr. Carlson was the Financial Controller of Integral Associates, LLC, a retail and wholesale cannabis operator in the State of Nevada, from 2018 to 2019, where he assisted in the sale of this company to a public company in the cannabis industry. Between 2017 and 2018, Mr. Carlson served as the Director of Finance at 777 U.S. Inc., the owner of a casino, restaurant and hotel in Carson City Nevada. From 2016 until 2017, he was the Controller at Basic Management, Inc., a real estate developer located in Henderson, Nevada. Between 2015 and 2016, Mr. Carlson was a consultant and the acting CFO of Gaming Ventures of Las Vegas, Inc., a privately held casino known as Club Fortune Casino. From 2012 through 2015, Mr. Carlson served as the Director of Accounting at the law firm of Lionel Sawyer & Collins. Mr. Carlson earned a Bachelor of Science in Business Administration with a major in Accounting from the University of Nevada and is a Certified Public Accountant.
Benjamin Sillitoe, Chief Executive Officer of CLS Nevada, Inc.
Mr. Sillitoe, was appointed to serve as Chief Executive Officer of CLS Nevada, Inc. commencing on July 1, 2018. Mr. Sillitoe co-founded Oasis Cannabis Center, LLC, a premier cannabis dispensary, in 2014 where he first served as the Finance Director and then as its CEO beginning in 2015. CLS acquired all of the membership interests in Alternative Solutions, L.L.C., the owner of Oasis Cannabis Center, LLC, in June 2018. Mr. Sillitoe has been a leader in the local Las Vegas cannabis industry since its inception, having served on the Board of Directors for the Nevada Dispensary Association, the largest cannabis trade association in Nevada, for over two years. Between 2012 and 2014, Mr. Sillitoe was the Finance Director of Proficio Mortgage, a subsidiary of Proficio Bank. Mr. Sillitoe earned a Bachelor of Science in Business Administration with a major in Managerial Finance from the University of Las Vegas.
Don Decatur, Chief Operating Officer of CLS Nevada, Inc.
Mr. Decatur was appointed to serve as the Chief Operating Officer of CLS Nevada, Inc. commencing on July 1, 2018. Prior to this appointment, Mr. Decatur was the Director of Operations of Alternative Solutions, L.L.C., which CLS acquired in June 2018. Between 2015 and 2016, Mr. Decatur was the Director of Product Development for Nevada Medical Group, LLC, d/b/a Body and Mind (BaM), a cannabis company. From 2010 until 2015, Mr. Decatur owned and served as CEO of SinCity Style, LLC, a cannabis merchandise and apparel company. Mr. Decatur has over 18 years of experience in the cannabis and horticulture business. He is responsible for the creation of numerous strains of cannabis, has won numerous industry awards, and has been honored by High Times magazine for creating two “Top Ten Strains of the Year”.
Our amended and restated articles of incorporation provide that the board of directors be divided into three classes with each class serving a staggered three-year term. The term of Class I directors expired at our 2018 annual meeting, the term of Class II directors expired at our 2016 annual meeting, and the term of Class III directors expired at our 2017 annual meeting. Andrew Glashow serves as the sole member of Class I, Frank Koretsky serves as the sole member of Class II and Jeffrey Binder serves as the sole member of Class III. We did not hold a 2015, 2016, 2017 or 2018 annual meeting due to our desire to conserve cash and focus on financing the Company. As a result, all of our directors are continuing to serve as directors because their successors have not been elected. We anticipate that we will hold an annual meeting during 2019 to elect directors. Executive officers are appointed by the board of directors and serve at its pleasure. None of our directors is independent, as that term is defined by Nasdaq rules. None of our directors is a financial expert, as that term is defined by the SEC.
We are not currently listed on any U.S. national securities exchange or quoted on an inter-dealer quotation system that has a requirement that certain of the members of the board of directors be independent. In evaluating the independence of its members and the composition of its planned committees, the board of directors utilizes the definition of “independence” developed by the Nasdaq Stock Market and in SEC rules, including the rules relating to the independence standards of audit committee members and the non-employee director definition of Rule 16b-3 promulgated under the Exchange Act. The board of directors has determined that none of its current members is independent.
The board of directors expects to continue to evaluate whether and to what extent the members of the board of directors are independent. The Company intends to appoint persons to the board of directors who will meet the corporate governance requirements imposed by a national securities exchange. Therefore, we expect that in the future a majority of our directors will be independent directors of which at least one director will qualify as an “audit committee financial expert,” within the meaning of SEC rules.
Additionally, the board of directors expects to appoint an audit committee, governance committee and compensation committee and to adopt charters relative to each such committee in the future.
Board of Directors and Corporate Governance
The board currently consists of three (3) members and is divided into three classes with each class of directors serving a staggered three-year term. Frank Koretsky’s term as a director expired in 2016, Jeff Binder’s term expired in 2017, and Andrew Glashow’s term expired in 2018, but each of them continues to hold office until his successor is elected.
Board Independence and Committees
We are not currently listed on any U.S. national securities exchange or quoted on an inter-dealer quotation system that has a requirement that certain of the members of the board of directors be independent. In evaluating the independence of its members and the composition of its planned committees, the board of directors utilizes the definition of “independence” developed by the Nasdaq Stock Market and in SEC rules, including the rules relating to the independence standards in audit committee members and the non-employee director definition of Rule 16b-3 promulgated under the Exchange Act. The board of directors has determined that none of its current members is independent.
The board of directors expects to continue to evaluate whether and to what extent the members of the board of directors are independent. The Company intends to appoint persons to the board of directors who will meet the corporate governance requirements imposed by a national securities exchange. Therefore, the Company expects that in the future a majority of our directors will be independent directors of which at least one director will qualify as an “audit committee financial expert,” within the meaning of SEC rules.
Additionally, the board of directors expects to appoint a governance committee and compensation committee and to adopt charters relative to each such committee in the future.
Code of Ethics
In order to conserve cash and because we only recently commenced earning revenue, we have not adopted a written code of ethics. Nevertheless, the board of directors expects to adopt a code of ethics shortly that is reasonably designed to deter wrongdoing and promote honest and ethical conduct; provide full, fair, accurate, timely and understandable disclosure in public reports; comply with applicable laws; ensure prompt internal reporting of code violations; and provide accountability for adherence to the code.
Section 16(a) Beneficial Ownership Reporting Compliance
During the year ended May 31, 2019, Mr. Jeffrey I. Binder, one of our officers and directors, failed to file two Form 4s on a timely basis. One Form 4 reflected one transaction and the other reflected two transactions in our securities. In addition, during the year ended May 31, 2019, Mr. Frank Koretsky, one of our directors, failed to file two Form 4s on a timely basis. One Form 4 reflected one transaction and the other reflected two transactions in our securities. Also during the year ended May 31, 2019, Mr. Glashow, one of our officers and directors, failed to file one Form 4 on a timely basis. This Form 4 reflected one transaction. Finally, during the year ended May 31, 2019, Todd Swanson, the sole member of ILJ, LLC, a 10% stockholder, failed to file Form 3 on a timely basis, which reflected one transaction, and Navy Capital Green Fund, LP, a 10% stockholder, failed to file Form 3 and two Form 4s on a timely basis, each reflecting two transactions in our securities.
Item 11. Executive Compensation.
As a smaller reporting company, we are required to disclose the executive compensation of our named executive officers, which consist of the following individuals, for the fiscal years ended May 31, 2018 and May 31, 2019, respectively: (i) any individual serving as our principal executive officer or acting in a similar capacity, during the fiscal year ended May 31, 2019; (ii) the two other most highly compensated executive officers of the Company serving as executive officers at the end of the most recently completed fiscal year; and (iii) up to two additional individuals for whom disclosure would have been provided but for the fact that the individual was not serving as an executive officer at the end of the most recently completed fiscal year.
Summary Compensation Table
The following table discloses compensation paid or to be paid to our named executive officers for the fiscal years ended May 31, 2019 and May 31, 2018.
|
Name and Principal Position |
Fiscal Year |
Salary ($) |
Bonus ($) |
Stock Awards ($) |
Non-Equity Incentive Plan Compensation ($) |
Non-Qualified Deferred Compensation ($) |
All Other Compensation ($) |
Total ($) |
||||||||||||||||||||||
|
Jeffrey Binder, |
2019 |
150,000 | — | — | — | — | — | 150,000 | ||||||||||||||||||||||
|
Chairman, and Chief Executive Officer |
2018 |
150,000 | — | — | — | — | — | 150,000 | ||||||||||||||||||||||
|
Andrew Glashow, |
2019 |
58,333 | 26,938 | (2) | — | — | 353,000 | (3) | 438,271 | |||||||||||||||||||||
|
President and Chief Operating Officer (1) |
2018 |
-- | — | — | — | — | — | |||||||||||||||||||||||
|
Benjamin Sillitoe |
2019 |
138,750 | — | 325,417 | (5) | — | — | 464,167 | ||||||||||||||||||||||
| Chief Executive Officer of CLS Nevada, Inc. (4) |
2018 |
-- | — | — | — | — | — | |||||||||||||||||||||||
|
1 |
Mr. Glashow and the Company entered into an employment agreement on March 1, 2019 at which time he was appointed President and Chief Operating Officer. Mr. Glashow had previously served as a consultant to and director of the Company. |
|
|
|
|
2 |
Pursuant to his employment agreement, Mr. Glashow was granted 500,000 shares of restricted common stock with a fair value of $215,500, which fully vest in two equal annual installments on March 1, 2020 and on March 1, 2021, assuming that Mr. Glashow remains employed by the Company on such dates or has been removed by the Company prior to such vesting date or dates without cause. Amount represents the portion of this stock grant which was charged to operations on a straight-line basis during the year ended May 31, 2019. |
|
|
|
|
3 |
Amount represents payments made to Mr. Glashow as a consultant to the Company, including a bonus earned in connection with the closing of the Acquisition Agreement, in the amount of $250,000. |
|
|
|
|
4 |
Mr. Sillitoe and the Company entered into an employment agreement on July 31, 2018 and he was appointed Chief Executive Officer of CLS Nevada, Inc. effective on July 1, 2018. |
|
|
|
|
5 |
Pursuant to his employment agreement, Mr. Sillitoe was granted 500,000 shares of restricted common stock with a fair value of $355,000, which fully vest on July 1, 2019. Amount represents the portion of this stock grant which was charged to operations on a straight-line basis during the year ended May 31, 2019. |
Narrative Disclosure to Summary Compensation Table
We currently do not have a stock option plan or any other incentive plan that provides for compensation intending to serve as an incentive for performance except as provided in the employment agreements of Messrs. Binder, Sillitoe, and Glashow.
The following is a narrative discussion of our officers’ employment agreements that we believe is necessary to understand the information disclosed in the foregoing Summary Compensation Table with respect to fiscal years 2019 and 2018, and which relates to executive officers we hired after the end of the most recent fiscal year.
Employment Agreements
CLS Labs and Jeffrey Binder entered into a five-year employment agreement effective October 1, 2014. Under the agreement, Mr. Binder serves as CLS Labs’ Chairman, President and Chief Executive Officer and is entitled to receive an annual salary of $150,000. Under the agreement, Mr. Binder is also entitled to receive a performance bonus equal to 2% of CLS Labs’ annual EBITDA, up to a maximum annual cash compensation of $1 million (including his base salary), and annual stock options, exercisable at the fair market value of CLS Labs’ common stock on the date of grant, in an amount equal to 2% of its annual EBITDA up to $42.5 million and 4% of its annual EBITDA in excess of $42.5 million.
On April 28, 2015, Mr. Binder, CLS Labs and the Company entered into an addendum to Mr. Binder’s employment agreement whereby Mr. Binder agreed that following the Merger, in addition to his obligations to CLS Labs, he would serve the Company and its subsidiaries in such roles as the Company may request. In exchange, the Company agreed to assume the obligations of CLS Labs to grant Mr. Binder annual stock options, as referenced above. Mr. Binder continues to receive an annual salary of $150,000 from CLS Labs for serving as its Chairman, President and Chief Executive Officer. Mr. Binder deferred all of the $250,000 in salary payable to him under his employment agreement through May 31, 2016. On July 20, 2016 and March 31, 2017, we issued Mr. Binder convertible promissory notes in exchange for $250,000 and $112,500 in deferred salary, respectively, among other amounts owed to Mr. Binder by the Company.
Effective November 30, 2017, the Company and Mr. Lamadrid entered into a one-year employment agreement. Pursuant to the agreement, Mr. Lamadrid commenced serving as the Company’s President and Chief Financial Officer on December 1, 2017. Under the agreement, Mr. Lamadrid is entitled to receive an annual salary of $175,000. Further, he is entitled to receive a performance bonus equal to 2% of the Company’s annual EBITDA, and annual restricted stock awards of the Company’s common stock in an amount equal to 3% of its annual EBITDA. Additionally, Mr. Lamadrid is entitled to a one-time signing bonus of 500,000 shares of restricted common stock of the Company, which shall become fully vested one year from the effective date of the agreement.
On July 24, 2018, the Company and Mr. Lamadrid, its President and Chief Financial Officer, mutually agreed to terminate the employment agreement dated December 1, 2017 between CLS and Mr. Lamadrid effective July 13, 2018. Mr. Lamadrid resigned as President and Chief Financial Officer effective as of July 13, 2018. The parties further agreed that neither party would have any further obligations under the employment agreement after such date. In connection with such separation, we agreed, among other provisions, to issued Mr. Lamadrid 600,000 shares of our common stock in full satisfaction of all obligations we had or allegedly had to issue him common stock for any reason, including the obligation to issue him restricted stock under his employment agreement. We also agreed to release Mr. Lamadrid from his non-competition obligations under the Confidentiality, Non-Compete and Property Rights Agreement dated November 30, 2017 between the parties. The balance of the terms of the confidentiality agreement will remain in full force and effect.
On July 31, 2018, CLS Nevada, Inc. and Mr. Sillitoe entered into a one-year employment agreement. Pursuant to the agreement, Mr. Sillitoe commenced serving as CLS Nevada’s Chief Executive Officer effective July 1, 2018. Under the agreement, Mr. Sillitoe is entitled to receive an annual salary of $150,000. Further, he is entitled to receive a performance bonus equal to 2% of CLS Nevada’s annual EBITDA, and annual restricted stock awards of the Company’s’ common stock in an amount equal to 3% of CLS Nevada’s annual EBITDA. Additionally, Mr. Sillitoe is entitled to a one-time signing bonus of 500,000 shares of restricted common stock of the Company, which shall become fully vested one year from the effective date of this agreement assuming Mr. Sillitoe remains employed by CLS Nevada on such date. Effective July 1, 2018, and in connection with the employment agreement, Mr. Sillitoe and the Company entered into a Confidentiality, Non-Compete and Proprietary Rights Agreement. Pursuant thereto, Mr. Sillitoe agreed (i) not to compete with the Company or CLS Nevada during the term of his employment and, unless he is terminated without cause, for a period of one year thereafter, (ii) not to release or disclose the Company’s or CLS Nevada’s confidential information, and (iii) to assign the rights to all work product to CLS Nevada, among other terms.
On July 31, 2019, CLS Nevada and Mr. Sillitoe entered into an amendment to his employment agreement to effect the original intention of the parties that the bonus provided for in Mr. Sillitoe’s employment agreement shall be based on the financial performance of Alternative Solutions and not of CLS Nevada.
CLS Nevada and Mr. Decatur entered into a one-year employment agreement on July 31, 2018. Pursuant to the agreement, Mr. Decatur commenced serving as CLS Nevada’s Chief Operating Officer on July 1, 2018. Under the agreement, Mr. Decatur is entitled to receive an annual salary of $150,000. Further, he is entitled to receive a performance bonus equal to 2% of CLS Nevada’s annual EBITDA, and annual restricted stock awards of the Company’s common stock in an amount equal to 3% of CLS Nevada’s annual EBITDA. Additionally, Mr. Decatur is entitled to a one-time signing bonus of 50,000 shares of restricted common stock of the Company, which shall become fully vested one year from the effective date of the agreement assuming Mr. Decatur remains employed by CLS Nevada on such date. Effective July 1, 2018, and in connection with the employment agreement, Mr. Decatur and the Company entered into a Confidentiality, Non-Compete and Proprietary Rights Agreement. Pursuant thereto, Mr. Decatur agreed (i) not to compete with the Company or CLS Nevada during the term of his employment and, unless he is terminated without cause, for a period of one year thereafter, (ii) not to release or disclose the Company’s or CLS Nevada’s confidential information, and (iii) to assign the rights to all work product to CLS Nevada, among other terms.
On May 14, 2019, CLS Nevada and Mr. Decatur entered into an amendment to his employment agreement to extend the term of Mr. Decatur’s employment agreement by two years instead of relying on the automatic one-year renewal provision in the employment agreement.
On July 31, 2019, CLS Nevada and Mr. Decatur entered into a second amendment to his employment agreement to effect the original intention of the parties that the bonus provided for in Mr. Decatur’s employment agreement shall be based on the financial performance of Alternative Solutions and not of CLS Nevada.
On March 1, 2019, the Company and Mr. Glashow entered into a two-year employment agreement and Mr. Glashow commenced serving as the Company’s President and Chief Operating Officer. Under the agreement, Mr. Glashow is entitled to receive an annual salary of $175,000. Further, he is entitled to receive a performance bonus equal to 1% of our annual EBITDA, and annual restricted stock awards in an amount equal to 1% of our annual EBITDA. Additionally, Mr. Glashow is entitled to a one-time signing bonus of 500,000 shares of our restricted common stock, half of which shall vest on March 1, 2020, and half of which shall vest on March 1, 2021. Effective March 1, 2019, and in connection with the employment agreement, Mr. Glashow and the Company entered into a Confidentiality, Non-Compete and Proprietary Rights Agreement. Pursuant thereto, Mr. Glashow agreed (i) not to compete with us during the term of his employment and for a period of one year thereafter, (ii) not to release or disclose our confidential information, and (iii) to assign the rights to all work product to us, among other terms.
On April 8, 2019, Alternative Solutions and Mr. Carlson entered into a one-year employment agreement and Mr. Carlson commenced serving as Alternative Solutions’ Controller. Under the agreement, Mr. Carlson is entitled to receive an annual salary of $110,000, and received a one-time signing bonus of 50,000 shares of restricted common stock of the Company, which shall become fully vested one year from the effective date of this agreement assuming Mr. Carlson remains employed by the Company on such date. In connection with the employment agreement, Mr. Carlson and the Company entered into a Confidentiality, Non-Compete and Proprietary Rights Agreement. Pursuant thereto, Mr. Carlson agreed (i) not to compete with the Company or Alternative Solutions during the term of his employment and, unless he is terminated without cause, for a period of one year thereafter, (ii) not to release or disclose the Company’s or Alternative Solutions’ confidential information, and (iii) to assign the rights to all work product to the Company, among other terms. On May 1, 2019, the Company appointed Mr. Carlson as its Chief Financial Officer and Mr. Carlson will continue his employment with us pursuant to the terms of his employment agreement.
Outstanding Equity Awards at May 31, 2019
None of our named executive officers had any outstanding stock options or unvested equity awards as of May 31, 2019, except Mr. Glashow who had 500,000 shares of restricted stock (pursuant to his employment agreement) that vest in two equal annual installments commencing on March 1, 2020; Mr. Sillitoe who had 500,000 shares of restricted stock (pursuant to his employment agreement) that vest on July 1, 2019; and Mr. Decatur who had 50,000 shares of restricted common stock (pursuant to his employment agreement) that vest on July 1, 2019.
Director Compensation
To date, we have not paid our directors any compensation for services on our board of directors. Our directors are, however, entitled to receive compensation as determined by the board of directors. On July 24, 2018, we awarded Star Associates, LLC, a limited liability company owned by Andrew Glashow, a director (and current officer) of CLS, a cash payment in the amount of $250,000 and 700,000 restricted shares of CLS’ common stock in recognition of Mr. Glashow’s efforts, through Star Associates, in successfully assisting us over the past year in negotiating and obtaining the financing necessary to acquire Alternative Solutions.
Item 12. Security Ownership of Certain Beneficial Owners and Management and Related Stockholder Matters.
The following table sets forth information with respect to the beneficial ownership of our common stock as of August 19, 2019 by (i) each stockholder known by us to be the beneficial owner of more than 5% of our common stock, (ii) each of our directors and named executive officers, and (iii) all of our directors and executive officers as a group. Our only class of voting securities is our common stock. To our knowledge, none of the shares listed below is held under a voting trust or similar agreement. To our knowledge, there are no pending arrangements, including any pledges by any person of securities of the Company, the operation of which may at a subsequent date result in a change in control of the Company. There were 126,420,345 shares of common stock issued and outstanding on August 19, 2019.
Unless otherwise indicated in the following table, the address for each person named in the table is c/o CLS Holdings USA, Inc., 11767 S. Dixie Hwy, Suite 115, Miami, FL 33156. Pursuant to SEC rules, we have included shares of common stock that the person has the right to acquire within 60 days from August 19, 2019.
Officers and Directors
|
Title of Class |
|
Name of Beneficial Owner(1) |
|
Amount and Nature of Beneficial Ownership |
|
|
|
Percentage of Class |
|
||
|
Common Stock |
|
Jeffrey I. Binder |
|
|
8,962,415 |
(2) |
|
|
|
7.08 |
|
|
Common Stock |
|
Frank Koretsky |
|
|
20,115,933 |
(3) |
|
|
|
15.76 |
|
|
Common Stock |
|
Andrew Glashow |
|
|
1,200,000 |
(4) |
|
|
|
* |
|
|
Common Stock |
|
Benjamin Sillitoe |
|
|
2,104,947 |
(5) |
|
|
|
1.67% |
|
|
Common Stock |
|
Don Decatur |
|
|
105,147 |
(6) |
|
|
|
* |
|
|
|
|
All directors and executive officers as a group (6 persons) |
|
|
33,267,431 |
|
|
|
|
26.01 |
|
|
* Indicates ownership of less than 1% of the outstanding shares of the Company’s common stock. |
|
||||||||||
|
1 |
Except as otherwise indicated, to our knowledge, the persons named in this table have sole voting, investment and dispositive power with respect to all shares of common stock listed. Under the rules of the Securities and Exchange Commission, a person (or group of persons) is deemed to be a “beneficial owner” of a security if he or she, directly or indirectly, has or shares the power to vote or to direct the voting of such security, or the power to dispose of or to direct the disposition of such security. Accordingly, more than one person may be deemed to be a beneficial owner of the same security. A person is also deemed to be a beneficial owner of any security, which that person has the right to acquire within 60 days, such as options or warrants to purchase our common stock. |
|
2 |
Includes (i) 8,717,971 shares of our common stock directly held by Mr. Binder; and (ii) 244,444 shares acquirable upon exercise of warrants that are currently exercisable. |
|
|
|
|
3 |
Includes (i) 13,474,821 shares of our common stock directly held by Mr. Koretsky; (ii) 1,198,568 shares acquirable upon exercise of warrants that are currently exercisable; and (iii) 5,442,544 shares of our common stock held of record by Newcan. Mr. Koretsky is the beneficial owner and has voting and investment power over the securities held by Newcan. |
|
|
|
|
4 |
Includes (i) 500,000 restricted shares of our common stock held by Mr. Glashow which vest in two equal annual installments commencing on March 1, 2020, assuming that Mr. Glashow remains employed by us on such dates or has been removed by us prior to such vesting date or dates without cause; and (ii) 700,000 shares of our common stock held of record by Star Associates, LLC, an entity wholly owned by Mr. Glashow. Mr. Glashow is the beneficial owner of Star Associates, LLC and has voting and investment power over the securities held by Star Associates, LLC. |
|
|
|
|
5 |
Represents shares of our common stock directly held by Mr. Sillitoe. |
|
|
|
|
6 |
Represents shares of our common stock directly held by Mr. Decatur. |
5% or Greater Shareholders
|
Title of Class |
|
Name and Address of Beneficial Owner(1) |
|
Amount and Nature of Beneficial Ownership |
|
|
|
Percentage of Class |
|
||
|
Common Stock |
|
ILJ, LLC |
|
|
13,644,293 |
(2) |
|
|
|
10.79 |
% |
|
|
|
10120 W. Flamingo Rd., Suite 4333, Las Vegas, NV 89135 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Common Stock |
|
Navy Capital Green Fund, LP |
|
|
24,375,000 |
(3) |
|
|
|
17.01 |
% |
|
|
|
575 Lexington Avenue, 4th Floor, New York, NY 10002 |
|
|
|
|
|
|
|
|
|
|
Common Stock |
Navy Capital Green Co-Invest Fund, LLC |
12,500,000 |
(4) |
9.42 |
% |
||||||
|
575 Lexington Avenue, 4th Floor, New York, NY 10002 |
|||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Common Stock |
|
Jeffrey I. Binder |
|
|
8,962,415 |
(5) |
|
|
|
7.08 |
% |
|
|
|
Miami, FL 33156 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Common Stock |
|
Frank Koretsky |
|
|
20,115,933 |
(6) |
|
|
|
15.76 |
% |
|
|
|
Sunny Isles Beach, FL 33160 |
|
|
|
|
|
|
|
|
|
|
1 |
Under the rules of the Securities and Exchange Commission, a person (or group of persons) is deemed to be a “beneficial owner” of a security if he or she, directly or indirectly, has or shares the power to vote or to direct the voting of such security, or the power to dispose of or to direct the disposition of such security. Accordingly, more than one person may be deemed to be a beneficial owner of the same security. A person is also deemed to be a beneficial owner of any security, which that person has the right to acquire within 60 days, such as options or warrants to purchase our common stock. Beneficial ownership does not include any shares the holder may receive upon the conversion of interest that has accrued or that will accrue in the future with respect to the convertible debentures. |
|
2 |
Represents shares of our common stock directly held by ILJ, LLC, an entity managed by Todd Swanson, a former manager of Alternative Solutions, which we acquired on June 27, 2018. |
|
|
|
|
3 |
Includes (i) 7,500,000 shares of our common stock; (ii) 7,500,000 shares issuable upon exercise of warrants at $0.60 per share; (iii) 6,250,000 shares acquirable upon conversion of convertible debentures; and (iv) 3,125,000 shares acquirable upon exercise of warrants at $1.10 per share. Navy Capital Green Fund LP is a Delaware limited partnership, of which Navy Capital Green Management, LLC, a New York limited liability company, is the investment manager. The investment manager has shared power with John Kaden and Sean Stiefel, the managers of the investment manager, to vote and dispose of the shares. |
|
4 |
Includes (i) 6,250,000 shares of our common stock; and (ii) 6,250,000 shares issuable upon conversion of warrants at $0.60 per share. Navy Capital Green Co-Invest Fund, LLC is a Delaware limited liability company, of which Navy Capital Green Management, LLC, a New York limited liability company, is the investment manager. The investment manager has shared power with John Kaden and Sean Stiefel, the managers of the investment manager, to vote and dispose of the shares. |
|
5 |
Includes (i) 8,717,971 shares of our common stock directly held by Mr. Binder; and (ii) 244,444 shares issuable upon exercise of warrants that are currently exercisable. |
|
|
|
|
6 |
Includes (i) 13,474,821 shares of our common stock directly held by Mr. Koretsky; (ii) 1,198,568 shares issuable upon exercise of warrants that are currently exercisable; and (iii) 5,442,544 shares of our common stock held of record by Newcan. Mr. Koretsky is the beneficial owner and has voting and investment power over the securities held by Newcan. |
We are not, to the best of our knowledge, directly or indirectly owned or controlled by another corporation or foreign government.
Change in Control
We are not aware of any arrangement that might result in a change in control in the future. We have no knowledge of any arrangements, including any pledge by any person of our securities, the operation of which may at a subsequent date result in a change in the Company’s control.
Item 13. Certain Relationships and Related Transactions, and Director Independence.
Related Party Transactions
David Lamadrid Note
On February 26, 2018, we entered into a securities purchase agreement with David Lamadrid, our former President and Chief Financial Officer, whereby Mr. Lamadrid agreed to purchase an 8% convertible promissory note in the aggregate principal amount of $31,250 (the “Lamadrid Note”) from us due, subject to the terms therein, eighteen (18) months from the date of issuance.
Mr. Lamadrid could, at his option, convert all or a portion of the Lamadrid Note and accrued but unpaid interest into shares of common stock at a conversion price of $0.3125 per share. On the closing date, we also issued Mr. Lamadrid a three-year common stock purchase warrant to purchase 25,000 shares of our common stock at an initial exercise price of $0.75 per share.
On August 21, 2018, we received a conversion notice from Mr. Lamadrid notifying us that he had converted $31,250 in principal and $1,247 of accrued interest into 103,989 shares of our common stock.
Koretsky and Affiliate Notes
Between August 11, 2015 and May 31, 2017, we borrowed an aggregate of $1,657,000 from Frank Koretsky, a director of the Company, and $150,000 from CLS CO 2016, LLC and $465,000 from Newcan Investment Partners, LLC, two entities that are affiliated with Mr. Koretsky. These loans were unsecured, accrued interest between 6% and 15% per year, were due either on demand or within three years after the date of the applicable note, and, in some cases, were convertible into shares of our common stock and warrants at rates between $0.25 and 1.07 per share. Effective on May 31, 2017, we entered into the Omnibus Loan Amendment Agreement, whereby the portion of these loans that was advanced prior to December 31, 2017 was converted into our common stock, together with accrued interest on these loans. As a result of these conversions, Mr. Koretsky, CLS CO 2016, LLC and Newcan converted an aggregate of $1,485,000, $150,000, and $460,000 in principal, and $130,069, 49,247 and $7,747 in accrued interest, into an aggregate of 6,460,276, 636,988 and 1,870,988 shares of common stock at $0.25 per share. Pursuant to the Omnibus Loan Amendment Agreement, the conversion rate on all of the loans made by Mr. Koretsky, CLS CO 2016, LLC, and Newcan was reduced, if applicable, to $0.25 per share and Mr. Koretsky and his affiliates gave up the right to receive warrants upon conversion. Thus, each of Mr. Koretsky, CLS CO 2016, LLC and Newcan received 4,560,849, 488,159 and 1,433,841 shares of common stock in excess of what they would have received had they converted their loans into common stock prior to the effective date of the Omnibus Loan Amendment Agreement.
Between June 1, 2017 and May 31, 2018, we borrowed an aggregate of $145,000 from Newcan Investment Partners, LLC, an entity that is affiliated with Mr. Koretsky. These loans were unsecured, accrued interest at 10% per year, were due either on demand or within three years after the date of the applicable note, and were convertible into shares of our common stock and warrants at $0.25 per share. On January 10, 2018, effective December 1, 2017, we entered into the Second Omnibus Loan Agreement with Newcan and Mr. Binder. The Second Omnibus Loan Agreement provides that the conversion price of all outstanding convertible promissory notes issued to Newcan as of the date of the agreement would be increased from $0.25 to $0.3125 per share of common stock. The remaining terms of such notes remain unchanged. Following the Second Omnibus Loan Agreement, on March 12, 2018, Newcan converted all of its outstanding convertible loans, which totaled $956,658 in principal and $98,098 in accrued interest, into a total of 3,375,220 shares of our common stock.
On August 6, 2018, we issued the Newcan Convertible Note 8 to finalize the terms of repayment with respect to a certain loan made to the Company by Newcan on May 4, 2018, which was converted into 196,336 shares of common stock on October 23, 2018.
Binder Notes
Between June 1, 2015 and May 31, 2017, we borrowed an aggregate of $251,800 from Jeffrey Binder, a director and officer of the Company. These loans were unsecured, accrued interest between 6% and 10% per year, were due either on demand or within three years after the date of the applicable note, and, in some cases, were convertible into shares of our common stock and warrants at rates between $.25 and 1.07 per share. Effective on May 31, 2017, we entered into the Omnibus Loan Amendment Agreement, whereby the portion of these loans that was advanced prior to May 31, 2017 was converted into our common stock, together with accrued interest on these loans. As a result of these conversions, Mr. Binder converted an aggregate of $442,750 in principal and $19,427 in accrued interest, into an aggregate of 1,848,708 shares of common stock at $.25 per share. Pursuant to the Omnibus Loan Amendment Agreement, the conversion rate on all of the loans made by Mr. Binder was reduced, if applicable, to $.25 per share and Mr. Binder gave up the right to receive warrants upon conversion. Thus, Mr. Binder received 1,127,061 shares of common stock in excess of what he would have received had he converted his loans into common stock prior to the effective date of the Omnibus Loan Amendment Agreement.
Between June 1, 2017 and March 31, 2018, we borrowed an aggregate of $204,881 from Mr. Binder. These loans were unsecured, accrued interest at 10% per year, were due either on demand or within three years after the date of the applicable note, and were convertible into shares of our common stock and warrants at $0.25 per share. On January 10, 2018, effective December 1, 2017, we entered into the Second Omnibus Loan Agreement with Newcan and Mr. Binder. The Second Omnibus Loan Agreement provides that the conversion price of all outstanding convertible promissory notes issued to Mr. Binder as of the date of the agreement would be increased from $0.25 to $0.3125 per share of common stock. The remaining terms of such notes remain unchanged. Following the Second Omnibus Loan Agreement, on March 12, 2018, Mr. Binder converted all of his outstanding convertible loans, which totaled $464,698 in principal and $43,058 in accrued interest, into a total of 1,624,819 shares of our common stock.
On April 6, 2018, we issued Binder Convertible Note 9, in the amount of $37,500.00, to Mr. Binder with respect to certain compensation payable to Mr. Binder as of February 28, 2018, which was repaid in full on August 7, 2018.
Omnibus Loan Amendment Agreement and Second Omnibus Loan Amendment
On May 31, 2017, we entered into an Omnibus Loan Amendment Agreement (the “Omnibus Loan Amendment”) with Jeffrey I. Binder, Frank Koretsky, Newcan Investment Partners LLC and CLS CO 2016, LLC (collectively, the “Insiders”). Pursuant to the Omnibus Loan Amendment, we agreed with the Insiders to amend certain terms of loans the Insiders made to us for working capital purposes, which loans were initially demand loans, and, except for certain loans made in 2017, were later memorialized as convertible loans (the “Insider Loans”), in exchange for the agreement of the Insiders to convert all Insider Loans where funds were advanced prior to January 1, 2017, which total $2,537,750, plus $166,490 of accrued interest thereon, into an aggregate of 10,816,960 shares of our common stock, and forego the issuance of warrants to purchase our common stock upon conversion. This resulted in the issuance of an additional 7,609,910 shares compared to the original number of shares issuable upon conversion of the Insider Loans prior to the Omnibus Loan Agreement. We valued the shares at $0.125, which was the market price of our stock at the conversion date, and charged the amount of $951,239 to loss on modification of debt during the twelve months ended May 31, 2017.
We entered into the Omnibus Loan Amendment in order to ease the debt burden on us and prevent us from defaulting on the Insider Loans. Pursuant to the Omnibus Loan Amendment, the following amendments were made to the Insider Loans: (a) we reduced the conversion price on the Insider Loans from between $0.75 and $1.07 per share of common stock to $0.25 per share of common stock, in those cases where the conversion price was greater than $0.25, which reduced conversion price exceeds the closing price of our common stock during the last three months; (b) we deleted the requirement to issue warrants to purchase our common stock upon conversion of the Insider Loans; (c) we amended one Insider Loan to permit conversion of only the portion of the Insider Loan related to services that were provided to us prior to January 1, 2017; and (d) we amended the terms of the Insider Loans where funds were advanced on or after January 1, 2017, which Insider Loans were not converted into our common stock, to provide for, where not already the case, a 10% interest rate per annum, a $0.25 conversion price per share of common stock, and the deletion of the requirement that we issue warrants to purchase our common stock upon conversion of such Insider Loans.
On January 10, 2018, effective December 1, 2017, we entered into the Second Omnibus Loan Agreement with Jeffrey I. Binder, an officer and director of the Company, and Newcan, an entity owned by Frank Koretsky, a director of the Company. The Second Omnibus Loan Agreement provides that the conversion price of all outstanding convertible promissory notes issued to either Mr. Binder or Newcan as of the date of the Agreement would be increased from $0.25 to $0.3125 per share of common stock. The remaining terms of such notes remain unchanged.
Agreement with Star Associates
On July 24, 2018, the Company awarded Star Associates, LLC, a limited liability company owned by Andrew Glashow, a director (and current officer) of the Company, a cash payment in the amount of $250,000 and 700,000 shares of restricted common stock in recognition of Mr. Glashow’s efforts, through Star Associates, in successfully assisting the Company in negotiating and obtaining the financing necessary to acquire Alternative Solutions, L.L.C.
Acquisition of Alternative Solutions
On June 27, 2018, we completed the purchase of all of the membership interests in Alternative Solutions and the Oasis LLCs from the members of such entities (other than Alternative Solutions) (the “Oasis Acquisition”). The closing occurred pursuant to a Membership Interest Purchase Agreement entered into between the Company and Alternative Solutions on December 4, 2017, as amended (the “Acquisition Agreement”). Pursuant to the Acquisition Agreement, we acquired all of the membership interests in Alternative Solutions, the parent of the Oasis LLCs, from its members, and the membership interests in the Oasis LLCs owned by members other than Alternative Solutions. In connection with the closing, Company employed Mr. Ben Sillitoe, the CEO and a member of Alternative Solutions, as the Chief Executive Officer of CLS Nevada, Inc., and Don Decatur, the COO and a member of the Oasis LLCs, as the Chief Operating Officer of CLS Nevada, Inc. Prior to the closing, Mr. Sillitoe owned 7.2940% of the membership interests in Alternative Solutions and Mr. Decatur owned 0.25% of the membership interests in each of the Oasis LLCs. Upon closing of the Oasis Acquisition, Messrs. Sillitoe and Decatur received $228,052.27 and $5,430.68, respectively, as consideration. Mr. Sillitoe is entitled to receive up to an additional $ 181,894 as consideration and Mr. Decatur is entitled to receive up to an additional $6,250 as consideration. Additionally, in connection with the Oasis Acquisition, we issued Messrs. Sillitoe 1,604,947 shares and Decatur 55,147 shares, respectively, of our common stock.
Item 14. Principal Accounting Fees and Services.
Audit Fees
Fees paid for audit services totaled approximately $58,000 during the year ended May 31, 2019. These amounts include fees associated with the annual audit of our financial and statutory statements, reviews of our quarterly financial statements and of our quarterly and annual reports on Form 10-Q and Form 10-K, respectively.
Fees paid for audit services totaled approximately $21,500 during the year ended May 31, 2018. These amounts include fees associated with the annual audit of our financial and statutory statements, reviews of our quarterly financial statements and of our quarterly and annual reports on Form 10-Q and Form 10-K, respectively.
Audit-Related Fees
We did not pay any fees for audit-related services in the year ended May 31, 2019.
We did not pay any fees for audit-related services in the year ended May 31, 2018.
Tax Fees
We did not pay any fees for tax-related services in the year ended May 31, 2019.
We did not pay any fees for tax-related services in the year ended May 31, 2018.
All Other Fees
We did not procure any other services from our auditors during the year ended May 31, 2019.
We did not procure any other services from our auditors during the year ended May 31, 2018.
PART IV
The following exhibits are included as part of this Registration Statement by reference:
|
Exhibit |
|
Description |
|
|
|
|
|
2.1 |
|
|
|
|
|
|
|
2.2 |
|
|
|
|
|
|
|
2.3 |
|
|
|
|
|
|
|
2.4 |
|
|
|
|
|
|
|
2.5 |
|
|
|
|
|
|
|
2.6 |
|
|
|
|
|
|
|
2.7 |
|
|
|
|
|
|
|
2.8 |
|
|
|
2.9* |
||
|
3.1 |
|
|
|
|
|
|
|
3.2 |
|
|
|
|
|
|
|
3.3 |
|
|
|
|
|
|
|
3.4 |
|
|
|
|
|
|
3.5 |
|
|
|
|
|
|
|
3.6 |
|
|
|
4.1 |
|
|
|
|
|
|
|
4.2 |
|
|
|
|
|
|
|
4.3 |
|
|
|
|
|
|
|
4.4 |
|
|
|
|
|
|
|
4.5 |
|
|
|
|
|
|
|
10.1 |
|
|
|
|
|
|
|
10.2 |
|
|
|
|
|
|
|
10.3 |
|
|
|
|
|
|
|
10.4 |
|
|
|
|
|
|
|
10.5** |
|
|
|
|
|
|
|
10.6** |
|
|
|
|
|
|
|
10.7 |
|
|
|
|
|
|
|
10.8 |
|
|
10.9 |
||
|
10.10 |
|
|
|
|
|
|
|
10.11 |
|
|
|
|
|
|
|
10.12 |
|
|
|
|
|
|
|
10.13 |
|
|
|
|
|
|
|
10.14 |
|
|
|
|
|
|
|
10.15 |
|
|
|
|
|
|
|
10.16 |
|
|
|
|
|
|
|
10.17 |
|
|
|
|
|
|
|
10.18 |
|
|
10.19 |
|
|
|
|
|
|
|
10.20 |
|
|
|
|
|
|
|
10.21 |
|
|
|
|
|
|
|
10.22 |
|
|
|
|
|
|
|
10.23 |
|
|
|
|
|
|
|
10.24 |
|
|
10.25 |
|
|
|
|
|
|
|
10.26 |
|
|
|
|
|
|
|
10.27 |
|
|
|
|
|
|
|
10.28 |
|
|
|
|
|
|
|
10.29 |
|
|
|
|
|
|
|
10.30 |
|
|
|
|
|
|
|
10.31 |
|
|
|
|
|
|
|
10.32 |
|
|
|
|
|
|
|
10.33 |
|
|
|
|
|
|
|
10.34 |
|
|
|
|
|
|
|
10.35 |
|
|
|
|
|
|
|
10.36 |
|
|
|
|
|
|
|
10.37 |
|
|
|
|
|
|
|
10.38 |
|
|
|
|
|
|
|
10.39 |
|
|
10.40 |
|
|
|
|
|
|
|
10.41 |
|
|
|
|
|
|
|
10.42 |
|
|
|
|
|
|
|
10.43 |
|
|
|
|
|
|
|
10.44 |
|
|
|
|
|
|
|
10.45 |
|
|
|
|
|
|
|
10.46 |
|
|
|
|
|
|
|
10.47 |
|
|
|
|
|
|
|
10.48 |
|
|
|
|
|
|
|
10.49 |
|
|
|
|
|
|
|
10.50 |
|
|
|
|
|
|
|
10.51 |
|
|
|
|
|
|
|
10.52 |
|
|
|
|
|
|
|
10.53 |
|
|
|
|
|
|
|
10.54 |
|
|
10.55 |
|
|
|
|
|
|
|
10.56 |
|
|
|
|
|
|
|
10.57 |
|
|
|
|
|
|
|
10.58 |
|
|
|
|
|
|
|
10.59 |
|
|
|
|
|
|
|
10.60 |
|
|
|
10.61* |
|
|
|
21.1* |
|
|
|
|
|
|
|
31.1* |
|
|
|
|
|
|
|
31.2* |
|
|
|
|
|
|
|
32.1* |
|
|
|
|
|
|
|
32.2* |
|
|
|
|
|
|
|
101.INS* |
|
XBRL Instance Document |
|
|
|
|
|
101.SCH* |
|
XBRL Taxonomy Extension Schema Document |
|
|
|
|
|
101.CAL* |
|
XBRL Taxonomy Extension Calculation Linkbase Document |
|
|
|
|
|
101.DEF* |
|
XBRL Taxonomy Extension Definition Linkbase Document |
|
|
|
|
|
101.LAB* |
|
XBRL Taxonomy Extension Label Linkbase Document |
|
|
|
|
|
101.PRE* |
|
XBRL Taxonomy Extension Presentation Linkbase Document |
(1) Management Contract or Compensation Plan
|
* |
Filed herewith. |
|
** |
Portions of this document are omitted pursuant to a confidential treatment order granted pursuant to Rule 24(b)-2 under the Exchange Act. Confidential portions of this document have been filed separately with the Securities and Exchange Commission. |
Pursuant to the requirements of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned thereunto duly authorized.
|
|
CLS HOLDINGS USA, INC. |
|
|
|
|
|
|
Date: August 29, 2019 |
By: |
/s/ Jeffrey I. Binder |
|
|
|
Jeffrey I. Binder |
|
|
|
Chairman and Chief Executive Officer (Principal Executive Officer) |
|
|
|
|
|
|
|
|
|
Date: August 29, 2019 |
By: |
/s/ Gregg Carlson |
|
|
|
Gregg Carlson |
|
|
|
Chief Financial Officer |
|
|
|
(Principal Financial and Accounting Officer) |
|
|
|
|
Pursuant to the requirements of the Securities Exchange Act of 1934, this report has been signed below by the following persons on behalf of the Registrant and in the capacities and on the dates indicated.
|
Name and Signature |
|
Title |
|
Date |
|
|
|
|
|
|
|
|
|
|
|
|
|
/s/ Jeffrey I. Binder |
|
Chairman, Chief Executive Officer and Director |
|
August 29, 2019 |
|
Jeffrey I. Binder |
|
(Principal Executive Officer) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
/s/ Gregg Carlson |
|
Chief Financial Officer |
|
August 29, 2019 |
|
Gregg Carlson |
|
(Principal Financial and Accounting Officer) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
/s/ Frank Koretsky |
|
Director |
|
August 29, 2019 |
|
Frank Koretsky |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
/s/ Andrew Glashow |
|
President, Chief Operating Officer and Director |
|
August 29, 2019 |
|
Andrew Glashow |
|
|
|
|